Carboxamide
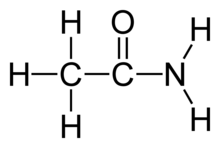
Acetamide, a simple carboxamide
In organic chemistry carboxamides (or amino carbonyls) are functional groups with the general structure R-CO-NR'R′′ with R, R', and R′′ as organic substituents, or hydrogen.[1]
Two amino acids, asparagine and glutamine, have a carboxamide group in them. The properties and reactivity of the carboxamide group arise from the hydrogen bonding capabilities of the -NH2 group as well as the carbonyl oxygen. Furthermore, the carbon atom in a carboxamide has a low-lying LUMO that is capable of accepting electron density from the nonbonding lone pair on the nitrogen, weakening the carbon-oxygen bond.
Examples of simple carboxamides include:
See also
References
- ↑ "Chapter 21: Amides and Imides". Nomenclature of Organic Compounds. Advances in Chemistry. Volume 126. pp. 166–173. doi:10.1021/ba-1974-0126.ch021. ISBN 9780841201910.
This article is issued from Wikipedia - version of the 10/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.