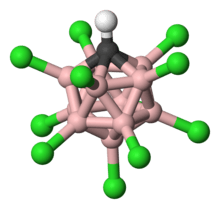
Carborane acid
Carborane acids H(CHB11X11) are a class of superacids[1] at least one million times stronger than 100% sulfuric acid in terms of their Hammett acidity function (H0) values, which measure the ability of a medium or solvent to donate protons.[2][3] The most well known example is chlorine based (X = Cl) [Figure 1]. The reason for their high acidities is that their conjugate bases, anions of the form (CHB
11X−
11), are highly delocalized and stable species substituted with electronegative groups (the (pseudo)halogen X). They are the only acids known to protonate C60 fullerene without decomposing it.[4][5] Additionally, they form stable, isolable salts with protonated benzene, C
6H+
7.
In coordination chemistry carboranes can be used as unique bulky ligand scaffolds. It was recently demonstrated that the same carboranyl moiety can act either as strongly electron-withdrawing or electron-donating substituent, depending on the positional attachment of the cluster to the heteroatom.[6]
The fluorinated carborane acid, H(CHB11F11), is even stronger than chlorinated carborane acid. It is able to protonate butane to form tert-butyl cation at room temperature and is the only known acid to protonate carbon dioxide to give the bridged cation, [H(CO2)2]+.[7][8][9][10]
Acidity
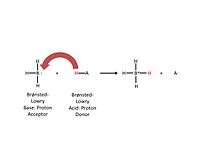
As defined in chemistry, a Brønsted-Lowry acid’s strength corresponds with its ability to release a hydrogen ion [Figure 2]. This superacid is known to be one million times stronger than sulfuric acid whose H0 value is −12.[11] However, the crystalline nature of these acids precludes direct measurement of this parameter. In terms of pKa, a slightly different measure of acidity defined as the ability of a given solute to undergo ionization in a solvent, carborane acids have pKa values well below −20 in 1,2-dichloroethane, with the (yet unknown) fully fluorinated analog H(CB11F12) having a calculated pKa of −46.[12]
Carborane acids differ from classical superacids in being well-defined one component substances. In contrast, classical superacids are often mixtures of a Brønsted acid and Lewis acid (e.g. HF/SbF5).[13] Despite being the strongest acid, the boron-based carborane acids are described as being "gentle", cleanly protonating weakly basic substances without further side reactions.[14] Whereas conventional superacids decompose fullerenes due to their strongly oxidizing Lewis acidic component, carborane acid has the ability to protonate fullerenes at room temperature to yield an isolable salt.[15][16] This property is also what makes carborane acid the first superacid that can be stored in a bottle.[17] Other comparable superacids, such as fluorosulfuric acid or hydrofluoric acid, readily donate protons in a similar way to carborane acid, but leave behind incredibly corrosive conjugate bases that are highly reactive and can even degrade glass by breaking down the protonated O–Si–O bond.[15] Carborane acid's remarkably stable conjugate base allows it to be contained easily because it does not react in this manner.[17]
History
Carborane acid was first discovered and synthesized by Professor Christopher Reed and his colleagues in 2004 at the University of California, Riverside.[16] Prior to carborane acid's discovery, the long-standing record of “strongest acids as single isolable compounds” was held by the two superacids, fluorosulfonic acid and trifluoromethanesulfonic acid, with pKas of −14 and −16 respectively.[18] The parent molecule from which carborane acid is derived, an icosahedral carborane anion, HCB
11H−
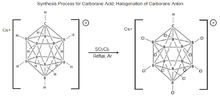
11, was first synthesized at DuPont in 1967 by Walter Knoth. Research into this molecules properties was put on hiatus until the mid 1980s when the Czech group of boron scientists, Plešek, Štíbr, and Heřmánek improved the process for halogenation of carborane molecules. These findings were instrumental in developing the current procedure for carborane acid synthesis.[15][18] The process [Figure 3] consists of treating CsHCB
11H
11 with SO
2Cl
2, refluxing under dry argon to fully chlorinate the molecule yielding carborane acid.[15][19]
Structure
Carborane acid consists of 11 boron atoms; each boron atom is bound to a chlorine atom. The chlorine atoms serve to enhance acidity and act as shields against attacks from the outside due to the steric hindrance they form around the cluster. The cluster, consisting of the 11 borons, 11 chlorines, and a single carbon atom, is paired with a hydrogen atom [Figure 1], bound to the carbon atom. The boron and carbon atoms are allowed to form six bonds due to boron’s ability to form a special kind of bond called “three-center-two-electron bond” [18] [Figure 4].
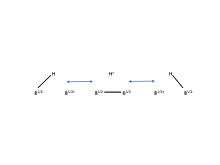 Figure 4. Boron has the ability to form a special kind of bond called “three-center-two-electron bond.” Pictured here are the resonance structures of a 3c-2e bond in diborane.
Figure 4. Boron has the ability to form a special kind of bond called “three-center-two-electron bond.” Pictured here are the resonance structures of a 3c-2e bond in diborane.
Though the structure of the carborane acid differs greatly from conventional acids, both distribute charge and stability in a similar fashion. The carborane anion distributes its charge by delocalizing the electrons throughout the 12 cage atoms.[20] This was shown in a single crystal X-ray diffraction study revealing shortened bond lengths in the heterocyclic portion of the ring suggesting electronic delocalization.[21]
The chlorinated carba-closo-dodecaborate anion HCB
11Cl−
11 is an outstandingly stable anion with what has previously been described as “substitutionally inert” B–Cl vertices [8] [Figure 5].
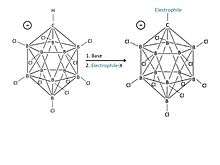
The -closo- term is designated to the molecule as a result of its closed shell electron count for boron chemistry.[22] The cagelike structure formed by the 11 boron atoms allows the electrons to be highly delocalized through the 3D cage, and the high energy required to disrupt the boron cluster portion of the molecule is what gives the anion its remarkable stability.[22] Because the anion is extremely stable, it will not easily take electrons from other atoms, which is the usual cause for acidic “carnage.” [23][24]
Usage
There are many uses for the boron-based carborane acid. The first is that it can add an octane kick to gasoline through a process called hydrocarbon cracking. Hydrocarbon cracking involves the reaction between acids and hydrocarbons from oil. This reaction increases the octane levels of gasoline by breaking the oil molecular into octane. The second usage of carborane acids is that it can help make vitamins digestible due to its strong acidity.[24] The acid plays an important role in digestion by activating digestive enzymes that ingest the protein, sugar or fat. Because of its stability and unreactivity, it will flood a solution with protons, allowing molecules to be frozen at crucial intermediate points. Carborane acids also play a key role in the manufacture of pharmaceutical drugs and petroleum products by delivering very clean acidity without ferocity.[25] A potential future application of carborane acid is the prospect of achieving "the holy grail of superacid chemistry," which is the protonation of the inert noble gas xenon.[26] Professor Reed wants to focus his further explorations of carborane acid's potential in this direction because "[ionizing Xe] has never been done before" and being able to react with such an inert molecule will have important implications for future use and new discoveries in inorganic chemistry.[17][18]
References
- ↑ Note that the image the acidic proton is not the one bonded to the carborane but that it is the counterion not displayed.
- ↑ Olah, G. A.; Prakash, G. K. S.; Sommer, J.; Molnar, A. (2009). Superacid Chemistry (2nd ed.). Wiley. p. 41. ISBN 978-0-471-59668-4.
- ↑ That is, the concentration of H+ in a solution of the carborane superacid is a million times higher than in a solution of sulfuric acid.
- ↑ Juhasz, M.; Hoffmann, S.; Stoyanov, E.; Kim, K.-C.; Reed, C. A. (2004). "The Strongest Isolable Acid". Angewandte Chemie International Edition. 43 (40): 5352–5355. doi:10.1002/anie.200460005. PMID 15468064.
- ↑ Reed, C. A. (2005). "Carborane acids. New "strong yet gentle" acids for organic and inorganic chemistry" (pdf). Chemical Communications. 2005 (13): 1669–1677. doi:10.1039/b415425h. PMID 15791295.
- ↑ Spokoyny, A. M.; Machan, C. W.; Clingerman, D. J.; Rosen, M. S.; Wiester, M. J.; Kennedy, R. D.; Stern, C. L.; Sarjeant, A. A.; Mirkin, C. A. (2011). "A coordination chemistry dichotomy for icosahedral carborane-based ligands". Nature Chemistry. 3 (8): 590–596. Bibcode:2011NatCh...3..590S. doi:10.1038/nchem.1088. PMID 21778977.
- ↑ http://onlinelibrary.wiley.com/doi/10.1002/anie.201308586/abstract
- ↑ Reed CA (2013). "Myths about the proton. The nature of H+ in condensed media". Acc. Chem. Res. 46: 2567–75. doi:10.1021/ar400064q. PMC 3833890
 . PMID 23875729.
. PMID 23875729. - ↑ Reed CA (2013). "Myths about the proton. The nature of H+ in condensed media". Acc. Chem. Res. 46: 2567–75. doi:10.1021/ar400064q. PMC 3833890
 . PMID 23875729.
. PMID 23875729. - ↑ Cummings, Steven; Hratchian, Hrant P.; Reed, Christopher A. (2016-01-22). "The Strongest Acid: Protonation of Carbon Dioxide". Angewandte Chemie International Edition. 55 (4): 1382–1386. doi:10.1002/anie.201509425. ISSN 1521-3773.
- ↑ Olah, G. A.; Prakash, G. K. S.; Sommer, J.; Molnar, A. (2009). Superacid Chemistry (2nd ed.). Wiley. p. 41. ISBN 978-0-471-59668-4.
- ↑ Lipping, Lauri; Leito, Ivo; Koppel, Ivar; Krossing, Ingo; Himmel, Daniel; Koppel, Ilmar A. (2015-01-14). "Superacidity of closo -Dodecaborate-Based Brønsted Acids: a DFT Study". The Journal of Physical Chemistry A. 119 (4): 735–743. doi:10.1021/jp506485x.
- ↑ Reed, Christopher. “Carborane acids. “New ‘strong yet gentle’ Acids For Organic and Inogronic Chemistry.” Advance Article (February 2005). Accessed February 13, 2015.
- ↑ Reed, Christopher A. "The Strongest Acid." Chemistry in New Zealand (October 2011): 174-179. Accessed February 13, 2015.
- 1 2 3 4 Juhasz M.; Hoffmann S.; Stoyanov E.; Kim K.-C.; Reed C. A. (2004). "The Strongest Isolable Acid". Angewandte Chemie International Edition. 43 (40): 5352–5355. doi:10.1002/anie.200460005. PMID 15468064.
- 1 2 Reed C. A. (2005). "Carborane acids. "New 'strong yet gentle' Acids For Organic and Inorganic Chemistry". Chemical Communications. 2005 (13): 1669–1677. doi:10.1039/b415425h. PMID 15791295.
- 1 2 3 Hopkin, M. (2004, November 1). World's strongest acid created. Retrieved March 3, 2015, from http://www.nature.com/news/2004/041115/full/news041115-5.html
- 1 2 3 4 Sato Kentaro, "The World’s Strongest Acid". The Museum of Organic Chemistry. Accessed February 13, 2015
- ↑ Gu, W., McCulloch, Billy J, Reibenspies, Joseph, and Ozerov, Oleg V. (2010, February 1). Chemical Communications Retrieved March 5, 2015, from http://pubs.rsc.org/en/content/articlepdf/2010/cc/c001555e
- ↑ El-Hellani A.; Lavallo V. (2014). "Fusing N-Heterocyclic Carbenes with Carborane Anions". Angew. Chem. Int. Ed. 53: 4489–4493. doi:10.1002/anie.201402445.
- ↑ Allen L. Chan, Javier Fajardo, Jr., James H. Wright, II, Matthew Asay, and Vincent Lavallo, Inorganic Chemistry 2013 52 (21), 12308-12310
- 1 2 "Carboranes: A New Class of Weakly Coordinating Anions for Strong Electrophiles, Oxidants, and Superacids". Accounts of Chemical Research. 31: 133–139. doi:10.1021/ar970230r.
- ↑ "Convenient C-alkylation of the [HCB11Cl11]−carborane anion". Dalton Trans. 41: 7842–7844. doi:10.1039/C2DT12431A.
- 1 2 Kean, Sam. The Disappearing Spoon: And Other True Tales Of Madness, Love, And The History Of The World From The Periodic Table Of The Elements. New York: Back Bay Books, 2011. Print.
- ↑ Lovekin Kris. "Strong, Yet Gentle, Acid Uncovered". University of California, Riverside. (November, 2004). Accessed February 13, 2015.
- ↑ Stiles, D. (2007, September 1). Column: Bench monkey. Retrieved March 3, 2015, from http://www.rsc.org/chemistryworld/Issues/2007/September/ColumnBenchMonkey.asp