Canidae
| Canids[1] Temporal range: 39.75–0 Ma | |
|---|---|
 | |
| Major extant canid genera left-to-right, top-to-bottom: Canis, Cuon, Lycaon, Cerdocyon, Chrysocyon, Speothos, Vulpes, Nyctereutes, Otocyon and Urocyon | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Suborder: | Caniformia |
| Family: | Canidae G. Fischer de Waldheim, 1817[2] |
| Genera and species | |
|
See text | |
The biological family Canidae /ˈkænᵻdiː/ [3] is a lineage of carnivorans that includes domestic dogs, wolves, foxes, jackals, dingoes, and many other extant and extinct dog-like mammals. A member of this family is called a canid (/ˈkænᵻd/, /ˈkeɪnᵻd/).[4]
The cat-like feliforms and dog-like caniforms emerged within the Carnivoramorpha 43 million years before present.[5] The caniforms included the fox-like Leptocyon genus whose various species existed from 34 million years before present before branching 11.9 million YBP into Vulpini (foxes) and Canini (canines).[6]:174–5
Canids are found on all continents except Antarctica, having arrived independently or accompanied human beings over extended periods of time. Canids vary in size from the 2-m-long (6 ft 7 in) gray wolf to the 24-cm-long (9.4 in) fennec fox. The body forms of canids are similar, typically having long muzzles, upright ears, teeth adapted for cracking bones and slicing flesh, long legs, and bushy tails. They are mostly social animals, living together in family units or small groups and behaving cooperatively. Typically, only the dominant pair in a group breeds, and a litter of young is reared annually in an underground den. Canids communicate by scent signals and by vocalizations. They are very intelligent. One canid, the domestic dog, long ago entered into a partnership with humans and today remains one of the most widely kept domestic animals.
Taxonomy
All living canids as a group form a monophyletic relationship with the extinct borophagines through both groups having a bicuspid (two points) on the lower carnassial talonid, which gives this tooth an additional ability in mastication. This together with the development of a distinct entoconid cusp and the broadening of the talonid of the first lower molar, and the corresponding enlargement of the talon of the upper first molar and reduction of its parastyle distinguish these late Cenozoic canids and are the essential differences that identify their clade.[6]:p6
Phylogenetic relationships

Within the Canidae, the results of allozyme and chromosome analyses have previously suggested several phylogenetic divisions:
- The wolf-like canids, (genus Canis, Cuon and Lycaon) including the dog (Canis lupus familiaris), gray wolf (Canis lupus), red wolf (Canis rufus), eastern wolf (Canis lycaon), coyote (Canis latrans), golden jackal (Canis aureus), Ethiopian wolf (Canis simensis), black-backed jackal (Canis mesomelas), side-striped jackal (Canis adustus), dhole (Cuon alpinus), and African wild dog (Lycaon pictus).[7]
- The fox-like canids, which include the kit fox (Vulpes aelox), red fox (Vulpes vulpes), Cape fox (Vulpes chama), Arctic fox (Vulpes lagopus), and fennec fox (Vulpes zerda).[7]
- The South American canids, including the bush dog (Speothos venaticus), hoary fox (Lycalopex uetulus), crab-eating fox (Cerdocyon thous) and maned wolf.[7]
- Various monotypic taxa, including the bat-eared fox (Otocyon megalotis), gray fox (Urocyon cinereoargenteus), and raccoon dog (Nycteruetes procyonoides).[7]
| Canidae |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
DNA analysis shows that the first three form monophyletic clades. The wolf-like canids and the fox-like canids together form the tribe Canini.[8] Molecular data imply a North American origin of living Canidae some ten million years ago and an African origin of wolf-like canines (Canis, Cuon, and Lycaon), with the jackals being the most basal of this group. The South American clade is rooted by the maned wolf and bush dog, and the fox-like canids by the fennec fox and Blanford's fox. The grey fox and island fox are basal to the other clades, however this topological difference is not strongly supported.[9]
Evolution
The Canidae today includes a diverse group of some 34 species ranging in size from the maned wolf with its long limbs to the short-legged bush dog. Modern canids inhabit forests, tundra, savannahs and deserts throughout tropical and temperate parts of the world. The evolutionary relationships between the species have been studied in the past using morphological approaches but more recently, molecular studies have enabled the investigation of phylogenetic relationships. In some species, genetic divergence has been suppressed by the high level of gene flow between different populations and where the species have hybridized, large hybrid zones exist.[10]
Eocene epoch
Carnivorans evolved from miacoids about 55 million years ago (Mya) during the late Paleocene.[11] Some five million years later, the carnivorans split into two main divisions: caniforms (dog-like) and feliforms (cat-like). By 40 Mya, the first member of the dog family proper had arisen. Called Prohesperocyon wilsoni, its fossilized remains have been found in what is now the southwestern part of Texas. The chief features which identify it as a canid include the loss of the upper third molar (part of a trend toward a more shearing bite), and the structure of the middle ear which has an enlarged bulla (the hollow bony structure protecting the delicate parts of the ear). Prohesperocyon probably had slightly longer limbs than its predecessors, and also had parallel and closely touching toes which differ markedly from the splayed arrangements of the digits in bears.[12]
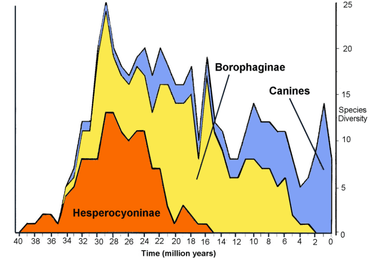
The canid family soon subdivided into three subfamilies, each of which diverged during the Eocene: Hesperocyoninae (about 39.74-15 Mya), Borophaginae (about 34-2 Mya), and Caninae (about 34-0 Mya). Caninae is the only surviving subfamily and all present-day canids including wolves, foxes, coyotes, jackals, and domestic dogs belong to it. Members of each subfamily showed an increase in body mass with time, and some exhibited specialised hypercarnivorous diets that made them prone to extinction.[13]:Fig. 1
Oligocene epoch
By the Oligocene, all three subfamilies of canids (Hesperocyoninae, Borophaginae, and Caninae) had appeared in the fossil records of North America. The earliest and most primitive branch of the Canidae was the Hesperocyoninae lineage, which included the coyote-sized Mesocyon of the Oligocene (38-24 Mya). These early canids probably evolved for the fast pursuit of prey in a grassland habitat; they resembled modern civets in appearance. Hesperocyonines eventually became extinct in the middle Miocene. One of the early members of the Hesperocyonines, the genus Hesperocyon, gave rise to Archaeocyon and Leptocyon. These branches led to the borophagine and canine radiations.[14]
Miocene epoch
Around 9–10 Mya during the Late Miocene, Canis, Urocyon, and Vulpes genera expanded from southwestern North America, where the canine radiation began. The success of these canines was related to the development of lower carnassials that were capable of both mastication and shearing.[14] Around 8 Mya, the Beringian land bridge allowed members of the genus Eucyon a means to enter Asia and they continued on to colonise Europe.[15]
Pliocene epoch
During the Pliocene, around 4–5 Mya, Canis lepophagus appeared in North America. This was small and sometimes coyote-like. Others were wolf-like in characteristics. Canis latrans (the coyote) is theorized to have descended from Canis lepophagus.[16]
The formation of the Isthmus of Panama, about 3 Mya, joined South America to North America, allowing canids to invade South America, where they diversified. However the most recent common ancestor of the South American canids lived in North America some 4 Mya and the likelihood is that there were more than one incursion across the new land bridge. One of the resulting lineages consisted of the gray fox (Urocyon cinereoargentus) and the now extinct dire wolf (Canis dirus). The other lineage consisted of the so-called South American endemic species, the maned wolf (Chrysocyon brachyurus), the short-eared dog (Atelocynus microtis), the bush dog (Speothos venaticus), the crab-eating fox (Cerdocyon thous) and the South American foxes (Lycalopex spp.). The monophyly of this group has been established by molecular means.[15]
Pleistocene epoch
During the Pleistocene, the North American wolf line appeared, with Canis edwardii, clearly identifiable as a wolf, and Canis rufus appeared, possibly a direct descendent of Canis edwardii. Around 0.8 Mya, Canis ambrusteri emerged in North America. A large wolf, it was found all over North and Central America, and was eventually supplanted by its descendant, the dire wolf, which then spread into South America during the late Pleistocene.[17]
By 0.3 Mya, a number of subspecies of the gray wolf (Canis lupus) had developed and had spread throughout Europe and northern Asia.[18] The gray wolf colonized North America during the late Rancholabrean era across the Bering land bridge, there being at least three separate invasions, with each one consisting of one or more different Eurasian gray wolf clades.[19] MtDNA studies have shown that there are at least four extant C. lupus lineages.[20] The dire wolf shared its habitat with the gray wolf but became extinct in a large-scale extinction event that occurred around 11,500 years ago. It may have been more of a scavenger than a hunter; its molars appear to be adapted for crushing bones and it may have died out as a result of the extinction of the large herbivorous animals on whose carcases it relied.[17]
In 2015, a study of mitochondrial genome sequences and whole genome nuclear sequences of African and Eurasian canids indicated that extant wolf-like canids have colonised Africa from Eurasia at least 5 times throughout the Pliocene and Pleistocene, which is consistent with fossil evidence suggesting that much of African canid fauna diversity resulted from the immigration of Eurasian ancestors, likely coincident with Plio-Pleistocene climatic oscillations between arid and humid conditions. When comparing the African and Eurasian golden jackals, the study concluded that the African specimens represented a distinct monophyletic lineage that should be recognized as a separate species, Canis anthus (African golden wolf). According to a phylogeny derived from nuclear sequences, the Eurasian golden jackal (Canis aureus) diverged from the wolf/coyote lineage 1.9 million years ago but the African golden wolf separated 1.3 million years ago. Mitochondrial genome sequences indicated the Ethiopian wolf diverged from the wolf/coyote lineage slightly prior to that.[21]:S1
Characteristics

Wild canids are found on every continent except Antarctica, and inhabit a wide range of different habitats, including deserts, mountains, forests, and grasslands. They vary in size from the fennec fox, which may be as little as 24 cm (9.4 in) in length and weigh 0.6 kg (1.3 lb),[22] to the gray wolf, which may be up to 160 cm (5.2 ft) long, and can weigh up to 79 kg (174 lb).[23] Only a few species are arboreal – the North American gray fox, the closely related Channel Island fox,[24] and the raccoon dog habitually climb trees.[25][26][27]
All canids have a similar basic form, as exemplified by the grey wolf, although the relative length of muzzle, limbs, ears and tail vary considerably between species. With the exceptions of the bush dog, raccoon dog, and some domestic breeds of Canis lupus, canids have relatively long legs and lithe bodies, adapted for chasing prey. The tails are bushy and the length and quality of the pelage varies with the season. The muzzle portion of the skull is much more elongated than that of the cat family. The zygomatic arches are wide, there is a transverse lambdoidal ridge at the rear of the cranium and in some species, a sagittal crest running from front to back. The bony orbits around the eye never form a complete ring and the auditory bullae are smooth and rounded.[28]
All canids are digitigrade, meaning they walk on their toes. The tip of the nose is always naked, as are the cushioned pads on the soles of the feet. These latter consist of a single pad behind the tip of each toe and a more-or-less three-lobed central pad under the roots of the digits. Hairs grow between the pads and in the Arctic fox, the sole of the foot is densely covered with hair at some times of year. With the exception of the four-toed African hunting dog (Lycaon pictus), there are five toes on the forefeet but the pollex (thumb) is reduced and does not reach the ground. On the hind feet, there are four toes, but in some domestic dogs, a fifth vestigial toe, known as a dewclaw, is sometimes present but has no anatomical connection to the rest of the foot. The slightly curved nails are non-retractile and more or less blunt.[28]
The penis in male canids is supported by a bone called the baculum. It also contains a structure at the base called the bulbus glandis which helps to create a copulatory tie during mating, locking the animals together for up to an hour.[29] Young canids are born blind, with their eyes opening a few weeks after birth.[30] All living canids (Caninae) have a ligament analogous to the nuchal ligament of ungulates used to maintain the posture of the head and neck with little active muscle exertion; this ligament allows them to conserve energy while running long distances following scent trails with their nose to the ground.[31] However, based on skeletal details of the neck, at least some Borophaginae (such as Aelurodon) are believed to have lacked this ligament.[31]
Dentition

Most canids have 42 teeth, with a dental formula of: 3.1.4.23.1.4.3. The bush dog has only one upper molar with two below, the dhole has two above and two below, and the bat-eared fox has three or four upper molars and four lower ones.[28] The molar teeth are strong in most species, allowing the animals to crack open bone to reach the marrow. The deciduous, or baby teeth, formula in canids is 3.1.33.1.3, molars being completely absent.[28]
Social behavior

Almost all canids are social animals and live together in groups. In general, they are territorial or have a home range and sleep in the open, using their dens only for breeding and sometimes in bad weather.[32] In most foxes, and in many of the true dogs, a male and female pair work together to hunt and to raise their young. Gray wolves and some of the other larger canids live in larger groups called packs. African wild dogs have packs which may consist of twenty to forty animals, and packs of fewer than about seven individuals may be incapable of successful reproduction.[33] Hunting in packs has the advantage that larger prey items can be tackled. Some species form packs or live in small family groups depending on the circumstances, including the type of available food. In most species, some individuals live on their own. Within a canid pack, there is a system of dominance so that the strongest, most experienced animals lead the pack. In most cases, the dominant male and female are the only pack members to breed.[34]
Canids communicate with each other by scent signals, by visual clues and gestures, and by vocalizations such as growls, barks, and howls. In most cases, groups have a home territory from which they drive out other conspecifics. The territory is marked by leaving urine scent marks, which warn trespassing individuals.[35] Social behaviour is also mediated by secretions from glands on the upper surface of the tail near its root and from the anal glands.[34]
Reproduction

Canids as a group exhibit several reproductive traits that are uncommon among mammals as a whole. They are typically monogamous, provide paternal care to their offspring, have reproductive cycles with lengthy proestral and dioestral phases and have a copulatory tie during mating. They also retain adult offspring in the social group, suppressing the ability of these to breed while making use of the alloparental care they can provide to help raise the next generation of offspring.[36]
During the proestral period, increased levels of oestradiol make the female attractive to the male. There is a rise in progesterone during the oestral phase and the female is now receptive. Following this, the level of oestradiol fluctuates and there is a lengthy dioestrous phase during which the female is pregnant. Pseudo-pregnancy frequently occurs in canids that have ovulated but failed to conceive. A period of anoestrus follows pregnancy or pseudo-pregnancy, there being only one oestral period during each breeding season. Small and medium-sized canids mostly have a gestation period of fifty to sixty days while larger species average sixty to sixty-five days. The time of year in which the breeding season occurs is related to the length of day, as has been demonstrated in the case of several species that have been translocated across the equator to the other hemisphere and experiences a six-month shift of phase. Domestic dogs and certain small canids in captivity may come into oestrus more frequently, perhaps because the photoperiod stimulus breaks down under conditions of artificial lighting.[36]
The size of a litter varies,with from one to sixteen or more pups being born. The young are born small, blind and helpless and require a long period of parental care. They are kept in a den, most often dug into the ground, for warmth and protection.[28] When the young begin eating solid food, both parents, and often other pack members, bring food back for them from the hunt. This is most often vomited up from the adult's stomach. Where such pack involvement in the feeding of the litter occurs, the breeding success rate is higher than is the case where females split from the group and rear their pups in isolation.[37] Young canids may take a year to mature and learn the skills they need to survive.[38] In some species, such as the African wild dog, male offspring usually remain in the natal pack, while females disperse as a group, and join another small group of the opposite sex to form a new pack.[39]
Inbreeding avoidance
Because the African wild dog (Lycaon pictus) largely exists in fragmented small populations, its existence is endangered. Inbreeding avoidance via mate selection is characteristic of the species and has important potential consequences for population persistence.[40] Inbreeding is rare within natal packs. Computer-population simulations indicate that all populations continuing to avoid incestuous mating will become extinct within 100 years due to the unavailability of unrelated mates.[40] Thus the impact of reduced numbers of suitable unrelated mates will likely have a severe demographic impact on the future viability of small wild dog populations.
Red wolves primarily live in packs composed of a socially monogamous breeding pair and offspring of different ages. Using long-term data on red wolf individuals of known pedigree, it was found that inbreeding among first-degree relatives was rare.[41] A likely mechanism for avoidance of inbreeding is independent dispersal trajectories from the natal pack. Many of the young wolves spend time alone or in small non-breeding packs composed of unrelated individuals. The union of two unrelated individuals in a new home range is the predominant pattern of breeding pair formation.[41]
Among Ethiopian wolves, most females disperse from their natal pack at about two years of age, and some become "floaters" that may successfully immigrate into existing packs. Breeding pairs are most often unrelated to each other, suggesting that female-biased dispersal reduces inbreeding.[42]
Grey wolves and Arctic foxes also exhibit inbreeding avoidance.[43]
Inbreeding is ordinarily avoided because it leads to a reduction in progeny fitness (inbreeding depression) due largely to the homozygous expression of deleterious recessive alleles.[44] Cross-fertilization between unrelated individuals ordinarily leads to the masking of deleterious recessive alleles in progeny.[45][46]
Inbreeding depression
On the basis of an analysis of data on 42,855 dachshund litters, it was found that as the inbreeding coefficient increased, litter size decreased and the percentage of stillborn puppies increased, thus indicating inbreeding depression.[47]
Canids and humans

One canid, the domestic dog, entered into a partnership with humans a long time ago. This partnership is documented as far back as 26,000 years ago, when the footprints of a young boy aged about eight to ten were found in Chauvet Cave in southern France, walking alongside what was identified as a large dog or wolf.[48] The earliest recorded fossil of a dog was found to be around 36,000 years ago in Goyet Cave in Belgium.[49] Even earlier, wolves were found fossilized in the same locations as humans at sites that date back 300,000 years, showing how far back humans and wolves had interactions with one another.[50] The fact that wolves are pack animals with cooperative social structures may have been the reason that the relationship developed. Humans benefited from the canid's loyalty, cooperation, teamwork, alertness and tracking abilities while the wolf may have benefited from the use of weapons to tackle larger prey and the sharing of food. Humans and dogs may have evolved together.[51] The bond between humans and dogs can be seen in the burial of dogs with their owners as early as 11,000 years ago in the Americas and 8,500 years ago in Europe.[50]
Among canids, only the gray wolf has widely been known to prey on humans.[52] Nonetheless, at least two records have coyotes killing humans,[53] and two have golden jackals killing children.[54] Human beings have trapped and hunted some canid species for their fur and, especially the gray wolf, coyote and the red fox, for sport.[55] Canids such as the dhole are now endangered in the wild because of persecution, habitat loss, a depletion of ungulate prey species and transmission of diseases from domestic dogs.[56]
Extant and recently extinct species



All extant species of family Canidae are in subfamily Caninae.
Subfamily Caninae
- True dogs – Tribe Canini
- Genus Canis (see also List of Canis species and subspecies which also includes some varieties)
- Gray wolf, Canis lupus (2.723 Mya to present)
- Domestic dog, Canis lupus familiaris
- Dingo, most often classified as Canis lupus dingo (sometimes considered a separate taxon)
- many other subspecies
- Red wolf, Canis rufus (sometimes considered a subspecies of gray wolf, but including several subtaxa of its own including the Florida black wolf)
- Coyote, Canis latrans (also called prairie wolf)
- African golden wolf, Canis anthus
- Golden jackal, Canis aureus
- Ethiopian wolf, Canis simensis (also called Abyssinian wolf, simien fox and simien jackal)
- Side-striped jackal, Canis adustus
- Black-backed jackal, Canis mesomelas
- Gray wolf, Canis lupus (2.723 Mya to present)
- Genus Cuon
- Dhole, Cuon alpinus or Canis alpinus (also called Asian wild dog)
- Genus Lycaon
- African wild dog, Lycaon pictus (also called African hunting dog)
- Genus Atelocynus
- Short-eared dog, Atelocynus microtis
- Genus Cerdocyon
- Crab-eating fox, Cerdocyon thous
- Genus Dusicyon †
- Falklands wolf, Dusicyon australis †
- Genus Lycalopex (Pseudalopex)
- Culpeo, Lycalopex culpaeus
- Darwin's fox, Lycalopex fulvipes
- South American gray fox, Lycalopex griseus
- Pampas fox, Lycalopex gymnocercus
- Sechura fox, Lycalopex sechurae
- Hoary fox, Lycalopex vetulus
- Genus Chrysocyon
- Maned wolf, Chrysocyon brachyurus
- Genus Speothos
- Bush dog, Speothos venaticus
- Genus Canis (see also List of Canis species and subspecies which also includes some varieties)
- True foxes – Tribe Vulpini
- Genus Vulpes
- Arctic fox, Vulpes lagopus
- Red fox, Vulpes vulpes (1 Mya to present) including a domesticated silver fox
- Swift fox, Vulpes velox
- Kit fox, Vulpes macrotis
- Corsac fox, Vulpes corsac
- Cape fox, Vulpes chama
- Pale fox, Vulpes pallida
- Bengal fox, Vulpes bengalensis
- Tibetan sand fox, Vulpes ferrilata
- Blanford's fox, Vulpes cana
- Rüppell's fox, Vulpes rueppelli
- Fennec fox, Vulpes zerda
- Genus Urocyon (2 Mya to present)
- Gray fox, Urocyon cinereoargenteus
- Island fox, Urocyon littoralis
- Cozumel fox, Urocyon sp.
- Genus Vulpes
- Basal Caninae
- Genus Otocyon (probably a vulpine close to Urocyon)
- Bat-eared fox, Otocyon megalotis
- Genus Nyctereutes
- Raccoon dog, Nyctereutes procyonoides
- Genus Otocyon (probably a vulpine close to Urocyon)
Prehistoric Canidae
Except where otherwise stated, the following classification is based on a 1994 paper by Xiaoming Wang, curator of terrestrial mammals at the Natural History Museum of Los Angeles County on the systematics of the subfamily Hesperocyoninae,[57] a 1999 paper by Wang, together with the zoologists Richard H. Tedford and Beryl E. Taylor on the subfamily Borophaginae,[58] and a 2009 paper by Tedford, Wang and Taylor on the North American fossil Caninae.[59]
Subfamily Caninae
- Tribe Canini[59]
- Genus Canis
- Canis adoxus †
- Canis ameghinoi †
- Canis apolloniensis (1.1 mya) †
- Canis armbrusteri (1.5 mya) †
- Canis arnensis (3.4 Mya, †)
- Canis cautleyi †
- Canis cedazoensis (4.6 mya) †
- Canis dirus (dire wolf), (0.25 mya) †
- Canis donnezani (4.0–3.1 Ma †, probably ancestor of wolves)
- Canis edwardii (4.6 mya) †, first species of wolf in North America)
- Canis (Eucyon) cipio (8.2 Mya †, probably first species of Canis genus)
- Canis etruscus (3.4 Mya †)
- Canis ferox (5 mya) †
- Canis gezi †
- Canis lepophagus (8 mya)†
- Canis michauxi †
- Canis mosbachensis (0.787 Mya †)
- Canis nehringi †
- Genus Cynotherium †
- Sardinian dhole, Cynotherium sardous †
- Genus Theriodictis (1.19 mya)†
- Genus Protocyon †
- Genus Dusicyon †
- Dusicyon avus †
- Genus Cerdocyon
- Cerdocyon avius †
- Cerdocyon ensenadensis †
- Genus Speothos
- Genus Nurocyon †
- Nurocyon chonokhariensis †
- Genus Xenocyon †
- Xenocyon falconeri (2.6 Mya †)
- Xenocyon lycaonoides (1.69 mya) †
- Genus Canis
- Tribe Vulpini
- Basal Caninae
- Genus Nyctereutes (7.1 Mya to present)
- First Caninae
Subfamily Borophaginae
† (Mya = million years ago) (million years = in existence)
- Tribe Phlaocyonini (27.2 million years) †
- Genus Cynarctoides (16.7 million years) †
- Cynarctoides acridens (11 million years) †
- Cynarctoides emryi (4 million years) †
- Cynarctoides gawnae (4 million years) †
- Cynarctoides harlowi (4 million years) †
- Cynarctoides lemur (30 Mya) †
- Cynarctoides luskensis (4.2 million years) †
- Cynarctoides roii (4.5 million years) †
- Genus Phlaocyon (30–19 Mya)
- Phlaocyon achoros
- Phlaocyon annectens (22 Mya)
- Phlaocyon latidens (30 Mya)
- Phlaocyon leucosteus (22 Mya)
- Phlaocyon mariae
- Phlaocyon marslandensis (19 Mya)
- Phlaocyon minor (30 Mya)
- Phlaocyon multicuspus
- Phlaocyon taylori[60]
- Phlaocyon yakolai (19 Mya)
- Genus Cynarctoides (16.7 million years) †
- Tribe Borophagini (16.7 million years) †
- Genus Cormocyon (10.2 million years) †
- Genus Desmocyon (9 million years) †
- Genus Metatomarctus (4.3 million years) †
- Genus Eulopocyon (18–16 Mya)
- Eulopocyon brachygnathus (16 Mya)
- Eulopocyon spissidens (18 Mya)
- Genus Psalidocyon (16 Mya)
- Psalidocyon marianae (16 Mya)
- Genus Microtomarctus (4 million years) †
- Genus Protomarctus (18 Mya)
- Protomarctus optatus (18 Mya)
- Genus Tephrocyon (16 Mya)
- Tephrocyon rurestris (16 Mya)
- Subtribe Cynarctina †
- Subtribe Aelurodontina (15 million years) †
- Subtribe Borophagina (17 million years) †
- Genus Paratomarctus (6 million years) †
- Paratomarctus euthos (13 Mya)
- Paratomarctus temerarius (16 Mya)
- Genus Carpocyon (19.7 million years) †
- Genus Protepicyon (16 Mya)
- Protepicyon raki (16 Mya)
- Genus Epicyon (2 million years) †
- Genus Borophagus (7 million years) †
- Borophagus diversidens (5 Mya) †
- Borophagus dudleyi (1.7 million years) †
- Borophagus hilli (6.7 million years) †
- Borophagus littoralis (0.6 million years) †
- Borophagus orc (0.4 million years) †
- Borophagus parvus (5.4 million years) †
- Borophagus pugnator (8.3 million years) †
- Borophagus secundus (8.3 million years) †
- Genus Paratomarctus (6 million years) †
Subfamily Hesperocyoninae
† (Mya = million years ago)
- Genus Cynodesmus (32–29 Mya)
- Cynodesmus martini (29 Mya)
- Cynodesmus thooides (32 Mya)
- ?Genus Caedocyon
- Caedocyon tedfordi
- Genus Ectopocynus (32–19 Mya)
- Ectopocynus antiquus (32 Ma)
- Ectopocynus intermedius (29 Mya)
- Ectopocynus siplicidens (19 Mya)
- Genus Enhydrocyon (29–25 Mya)
- Enhydrocyon basilatus (25 Mya)
- Enhydrocyon crassidens (25 Mya)
- Enhydrocyon pahinsintewkpa (29 Mya)
- Enhydrocyon stenocephalus (29 Mya)
- Genus Hesperocyon (39.74–34 Mya)
- Hesperocyon coloradensis
- Hesperocyon gregarius (37 Mya)
- Genus Mesocyon (34–29 Mya)
- Mesocyon brachyops (29 Mya)
- Mesocyon coryphaeus (29 Mya)
- Mesocyn temnodon
- Genus Osbornodon (32–18 Mya)
- Osbornodon brachypus
- Osbornodon fricki (18 Mya)
- Osbornodon iamonensis (21 Mya)
- Osbornodon renjiei (33 Mya)
- Osbornodon scitulus[62]
- Osbornodon sesnoni (32 Mya)
- Osbornodon wangi[60]
- Genus Paraenhydrocyon (30–25 Mya)
- Paraenhydrocyon josephi (30 Mya)
- Paraenhydrocyon robustus (25 Mya)
- Genus Philotrox (29 Mya)
- Philotrox condoni (29 Mya)
- Genus Prohesperocyon (36 Mya)
- Prohesperocyon wilsoni (36 Mya)
- Genus Sunkahetanka (29 Mya)
- Sunkahetanka geringensis (29 Mya)
- Genus Cynodesmus (32–29 Mya)
References
- ↑ Wozencraft, W.C. (2005). "Order Carnivora". In Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. ISBN 978-0-8018-8221-0. OCLC 62265494.
- ↑ Fischer de Waldheim, G. 1817. Adversaria zoological. Memoir Societe Naturelle (Moscow) 5:368–428. p372
- ↑ Canidae. Dictionary.com. The American Heritage Stedman's Medical Dictionary. Houghton Mifflin Company. http://dictionary.reference.com/browse/Canidae (accessed: February 16, 2009).
- ↑ Canid Merriam-Webster.com. Canid definition Merriam-Webster. Retrieved 2014-05-27
- ↑ Flynn, John J.; Wesley-Hunt, Gina D. (2005). "Phylogeny of the Carnivora: Basal Relationships Among the Carnivoramorphans, and Assessment of the Position of 'Miacoidea' Relative to Carnivora". Journal of Systematic Paleontology. 3: 1–28.
- 1 2 Tedford, R (2009). "Phylogenetic Systematics of the North American Fossil Caninae (Carnivora: Canidae)". Bulletin of the American Museum of Natural History. 325: 1–218. doi:10.1206/574.1.
- 1 2 3 4 Wayne, Robert K. (June 1993). "Molecular evolution of the dog family". Trends in Genetics. 9 (6): 218–224. doi:10.1016/0168-9525(93)90122-x. PMID 8337763.
- ↑ Jensen, Per (2007). The Behavioural Biology of Dogs. CABI. pp. 11–13. ISBN 978-1-84593-188-9.
- ↑ Lindblad-Toh, Kerstin; Wade, Claire M; Mikkelsen, Tarjei S.; Karlsson, Elinor K.; Jaffe, David B.; Kamal, Michael; Clamp, Michele; Chang, Jean L.; Kulbokas, Edward J.; Zody, Michael C.; Mauceli, Evan; Xie, Xiaohui; Breen, Matthew; Wayne, Robert K.; Ostrander, Elaine A.; Ponting, Chris P.; Galibert, Francis; Smith, Douglas R.; Dejong, Pieter J.; Kirkness, Ewen; Alvarez, Pablo; Biagi, Tara; Brockman, William; Butler, Jonathan; Chin, Chee-Wye; Cook, April; Cuff, James; Daly, Mark J.; Decaprio, David; et al. (2005). "Genome sequence, comparative analysis and haplotype structure of the domestic dog". Nature. 438 (7069): 803 in 803–19. doi:10.1038/nature04338. PMID 16341006.
- ↑ Wayne, Robert K. "Molecular evolution of the dog family". Retrieved 2014-05-27.
- ↑ "The Paleobiology Database". Paleodb.org. Retrieved 2012-06-12.
- ↑ Wang, Xiaoming (2008). "How Dogs Came to Run the World". Natural History Magazine. Vol. July/August. Retrieved 2014-05-24.
- ↑ Van Valkenburgh, B.; Wang, X.; Damuth, J. (Oct 2004). "Cope's Rule, Hypercarnivory, and Extinction in North American Canids". Science. 306 (5693): 101–104. Bibcode:2004Sci...306..101V. doi:10.1126/science.1102417. ISSN 0036-8075. PMID 15459388.
- 1 2 Martin, L.D. 1989. Fossil history of the terrestrial carnivora. Pages 536–568 in J.L. Gittleman, editor. Carnivore Behavior, Ecology, and Evolution, Vol. 1. Comstock Publishing Associates: Ithaca.
- 1 2 Perini, F. A.; Russo, C. A. M.; Schrago, C. G. (2010). "The evolution of South American endemic canids: a history of rapid diversification and morphological parallelism". Journal of Evolutionary Biology. 23 (2): 311–322. doi:10.1111/j.1420-9101.2009.01901.x. PMID 20002250.
- ↑ Nowak, R.M. 1979. North American Quaternary Canis. Monograph of the Museum of Natural History, University of Kansas 6:1 – 154.
- 1 2 Larson, Robert. "Wolves, coyotes and dogs (Genus Canis)". The Midwestern United States 16,000 years ago. Illinois State Museum. Retrieved 2014-06-07.
- ↑ Nowak, R. 1992. Wolves: The great travelers of evolution. International Wolf 2(4):3 – 7.
- ↑ Chambers, S. M.; Fain, S. R.; Fazio, B.; Amaral, M. (2012). "An account of the taxonomy of North American wolves from morphological and genetic analyses". North American Fauna. 77: 1–67. doi:10.3996/nafa.77.0001.
- ↑ Gaubert, P.; Bloch, C.; Benyacoub, S.; Abdelhamid, A.; Pagani, P.; et al. (2012). "Reviving the African Wolf Canis lupus lupaster in North and West Africa: A Mitochondrial Lineage Ranging More than 6,000 km Wide". PLoS ONE. 7 (8): e42740. doi:10.1371/journal.pone.0042740. PMC 3416759
 . PMID 22900047.
. PMID 22900047. - ↑ Koepfli, Klaus-Peter; Pollinger, John; Godinho, Raquel; Robinson, Jacqueline; Lea, Amanda; Hendricks, Sarah; Schweizer, Rena M.; Thalmann, Olaf; Silva, Pedro; Fan, Zhenxin; Yurchenko, Andrey A.; Dobrynin, Pavel; Makunin, Alexey; Cahill, James A.; Shapiro, Beth; Álvares, Francisco; Brito, José C.; Geffen, Eli; Leonard, Jennifer A.; Helgen, Kristofer M.; Johnson, Warren E.; o'Brien, Stephen J.; Van Valkenburgh, Blaire; Wayne, Robert K. (2015). "Genome-wide Evidence Reveals that African and Eurasian Golden Jackals Are Distinct Species". Current Biology. 25 (16): 2158–65. doi:10.1016/j.cub.2015.06.060. PMID 26234211.
- ↑ Marc Tyler Nobleman (2007). Foxes. Marshall Cavendish. p. 35. ISBN 978-0-7614-2237-2.
- ↑ Heptner, V. G.; Naumov, N. P. (1998), Mammals of the Soviet Union Vol.II Part 1a, Sirenia and Carnivora (Sea cows; Wolves and Bears), Science Publishers, Inc. USA., pp. 166–176, ISBN 1-886106-81-9
- ↑ "ADW: Urocyon littoralis: Information". Animaldiversity.ummz.umich.edu. 1999-11-28. Retrieved 2012-06-12.
- ↑ Kauhala, K.; Saeki, M. (2004). Raccoon Dog«. Canid Species Accounts. IUCN/SSC Canid Specialist Group. Pridobljeno 15 April 2009.
- ↑ Ikeda, Hiroshi (August 1986). "Old dogs, new tricks: Asia's raccoon dog, a venerable member of the canid family is pushing into new frontiers". Natural History. 95 (8): 40, 44.
- ↑ Raccoon dog – Nyctereutes procyonoides. WAZA – World Association of Zoos and Aquariums.
- 1 2 3 4 5 Mivart, St George Jackson (1890). Dogs, Jackals, Wolves, and Foxes: A Monograph of the Canidae. pp. xiv–xxxvi.
- ↑ Ewer, R. F. (1973). The Carnivores. Cornell University Press. ISBN 978-0-8014-8493-3.
- ↑ Macdonald, D. (1984). The Encyclopedia of Mammals. New York: Facts on File. p. 57. ISBN 0-87196-871-1.
- 1 2 Wang, Xiaoming; Tedford, Richard H. Dogs: Their Fossil Relatives and Evolutionary History. New York: Columbia University Press, 2008. pp. 97–8
- ↑ Harris, Stephen; Yalden, Derek (2008). Mammals of the British Isles (4th Revised ed.). Mammal Society. p. 413. ISBN 0-906282-65-9.
- ↑ McConnell, Patricia B. (2009-08-31). "Comparative canid behaviour". The other end of the leash. Retrieved 2014-06-12.
- 1 2 "Canidae: Coyotes, dogs, foxes, jackals, and wolves". Animal Diversity Web. University of Michigan. Retrieved 2014-06-13.
- ↑ Nowak, R. M.; Paradiso, J. L. 1983. Walker's Mammals of the World. Baltimore, Maryland: The Johns Hopkins University Press. ISBN 0-8018-2525-3.
- 1 2 Asa; Valdespino (2003). Carbyn, Ludwig N.; Sovada, Marsha Ann, ed. A review of Small Canid Reproduction: in The Swift Fox: Ecology and Conservation of Swift Foxes in a Changing World. University of Regina Press. pp. 117–123. ISBN 978-0-88977-154-3.
|first1=missing|last1=in Editors list (help);|first2=missing|last2=in Editors list (help) - ↑ Jensen, Per, ed. (2007). The Behavioural Biology of Dogs. CABI. pp. 158–159. ISBN 978-1-84593-188-9.
- ↑ Voelker, W. 1986. The Natural History of Living Mammals. Medford, New Jersey: Plexus Publishing. ISBN 0-937548-08-1
- ↑ "Lycaon pictus". Animal Info: Endangered animals of the world. 2005-11-26. Retrieved 2014-06-11.
- 1 2 Becker PA, Miller PS, Gunther MS, Somers MJ, Wildt DE, Maldonado JE (2012). "Inbreeding avoidance influences the viability of reintroduced populations of African wild dogs (Lycaon pictus)". PLoS ONE. 7 (5): e37181. doi:10.1371/journal.pone.0037181. PMC 3353914
 . PMID 22615933.
. PMID 22615933. - 1 2 Sparkman AM, Adams JR, Steury TD, Waits, LP, Murray DL. Pack social dynamics and inbreeding avoidance in the cooperatively breeding red wolf. Behavioral Ecology. 2012 July; 23(6):1186-1194. doi:10.1093/beheco/ars099
- ↑ Randall DA, Pollinger JP, Wayne RK, Tallents LA, Johnson PJ, Macdonald DW. Inbreeding is reduced by female-biased dispersal and mating behavior in Ethiopian wolves. Behavioral Ecology. 2007;18(3):579-89. doi:10.1093/beheco/arm010
- ↑ Geffen E, Kam M, Hefner R, Hersteinsson P, Angerbjörn A, Dalèn L, Fuglei E, Norèn K, Adams JR, Vucetich J, Meier TJ, Mech LD, Vonholdt BM, Stahler DR, Wayne RK (2011). "Kin encounter rate and inbreeding avoidance in canids". Mol. Ecol. 20 (24): 5348–58. doi:10.1111/j.1365-294X.2011.05358.x. PMID 22077191.
- ↑ Charlesworth D, Willis JH (2009). "The genetics of inbreeding depression". Nat. Rev. Genet. 10 (11): 783–96. doi:10.1038/nrg2664. PMID 19834483.
- ↑ Bernstein H, Hopf FA, Michod RE (1987). "The molecular basis of the evolution of sex". Adv. Genet. Advances in Genetics. 24: 323–70. doi:10.1016/s0065-2660(08)60012-7. ISBN 9780120176243. PMID 3324702.
- ↑ Michod, R.E. (1994). "Eros and Evolution: A Natural Philosophy of Sex" Addison-Wesley Publishing Company, Reading, Massachusetts. ISBN 0201442329 ISBN 978-0201442328
- ↑ Gresky C, Hamann H, Distl O (2005). "[Influence of inbreeding on litter size and the proportion of stillborn puppies in dachshunds]". Berl. Munch. Tierarztl. Wochenschr. (in German). 118 (3–4): 134–9. PMID 15803761.
- ↑ Derr, Mark (2011-10-29). "From the Cave to the Kennel". The Wall Street Journal. Archived from the original on 2013-11-22. Retrieved 2012-12-02.
- ↑ Shipman, Pat. (2009). "The Woof at the Door." (PDF). American Scientist. Archived from the original (PDF) on 2013-12-04. Retrieved 2012-12-02.
- 1 2 Galibert, F.; Quignon, P.; Hitte, C.; Andre, C. (2011). "Toward understanding dog evolutionary and domestication history" (PDF). Comptes Rendus Biologies. 334 (3): 190–196. doi:10.1016/j.crvi.2010.12.011. PMID 21377613. Archived from the original (PDF) on 2013-12-04. Retrieved 2012-12-02.
- ↑ Schleidt, Wolfgang M.; Shalter, Michael D. (2003). "Co-evolution of Humans and Canids: An Alternative View of Dog Domestication: Homo Homini Lupus?" (PDF). Evolution and Cognition. 9 (1): 57–72. Archived from the original (PDF) on 2014-04-11.
- ↑ Kruuk, H. 2002. Hunter and Hunted: Relationships between Carnivores and People. Cambridge, UK: Cambridge University Press. ISBN 0-521-81410-3.
- ↑ "Coyote Attacks: An Increasing Suburban Problem" (PDF). Archived from the original (PDF) on 2006-02-24. Retrieved 2007-08-19.
- ↑ "Canis aureus". Animal Diversity Web. University of Michigan. Retrieved 2007-07-31.
- ↑ "Fox hunting worldwide". BBC News. 1999-09-16. Retrieved 2014-06-16.
- ↑ Durbin, L.S.; Hedges, S.; Duckworth, J.W.; Tyson, M.; Lyenga, A.; Venkataraman, A. (2008). "Cuon alpinus". IUCN Red List of Threatened Species. Version 2013.2. International Union for Conservation of Nature. Retrieved 2014-06-16.
- ↑ Wang, Xiaoming (1994). "Phylogenetic systematics of the Hesperocyoninae". Bulletin of the American Museum of Natural History. 221: 1–207. hdl:2246/829.
- ↑ Wang, Xiaoming; Tedford, Richard H.; Taylor, Beryl E. (1999). "Phylogenetic systematics of the Borophaginae". Bulletin of the American Museum of Natural History. 243: 1–391. hdl:2246/1588.
- 1 2 Tedford, Richard; Wang, Xiaoming; Taylor, Beryl E. (2009). "Phylogenetic systematics of the North American fossil Caninae (Carnivora: Canidae)". Bulletin of the American Museum of Natural History. 325: 1–218. doi:10.1206/574.1.
- 1 2 Hayes, F.G. (2000). "The Brooksville 2 local fauna (Arikareean, latest Oligocene) Hernando County, Florida". Bulletin of the Florida Museum of Natural History. 43 (1): 1–47.
- ↑ Wang, Xiaoming; Wideman, Benjamin C.; Nichols, Ralph; Hanneman, Debra L. (2004). "A new species of Aelurodon (Carnivora, Canidae) from the Barstovian of Montana" (PDF). Journal of Vertebrate Paleontology. 24 (2): 445–452. doi:10.1671/2493. Archived from the original (PDF) on September 30, 2007. Retrieved 2007-07-08.
- ↑ Wang, Xiaoming (2003). "New Material of Osbornodon from the Early Hemingfordian of Nebraska and Florida" (PDF). Bulletin of the American Museum of Natural History. 279: 163–176. doi:10.1206/0003-0090(2003)279<0163:C>2.0.CO;2.
General references
- Xiaoming Wang, Richard H. Tedford, Mauricio Antón, Dogs: Their Fossil Relatives and Evolutionary History, New York : Columbia University Press, 2008; ISBN 978-0-231-13528-3
External links
| Wikispecies has information related to: Canidae |
| Wikimedia Commons has media related to Canidae. |
.jpg)
.jpg)





.jpg)
