Bufadienolide
 | |
| Names | |
|---|---|
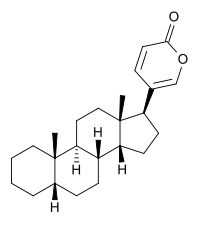
| IUPAC name
5-[(5R, 8R,9S,10S,13S,14S,17S)-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pyran-2-one | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:83977 |
| ChemSpider | 26286947 |
| PubChem | 3035030 |
| |
| |
| Properties | |
| C24H34O2 | |
| Molar mass | 354.53 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Bufadienolide is a chemical compound with steroid structure. Its derivatives are collectively known as bufadienolides, including many in the form of bufadienolide glycosides (bufadienolides that contain structural groups derived from sugars). These are a type of cardiac glycoside, the other being the cardenolide glycosides. Both bufadienolides and their glycosides are toxic; specifically, they can cause an atrioventricular block, bradycardia (slow heartbeat), ventricular tachycardia (a type of rapid heartbeat), and possibly lethal cardiac arrest.[1]
Etymology
The term derives from the toad genus Bufo that contains bufadienolide glycosides, the suffix -adien- that refers to the two double bonds in the lactone ring, and the ending -olide that denotes the lactone structure. Consequently, related structures with only one double bond are called bufenolides, and the saturated equivalent is bufanolide.[2]

Classification
According to MeSH, bufadienolides and bufanolides are classified as follows:[3][4]
- Polycyclic compounds
- Steroids
- Cardanolides
- Cardiac glycosides
- Bufanolides (includes bufenolides, bufadienolides, bufatrienolides)
- Cardenolides
- Cardiac glycosides
- Cardanolides
- Steroids
References
- ↑ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 534, 538. ISBN 3-8047-1763-2.
- ↑ IUPAC Recommendations 1999: Revised Section F: Natural Products and Related Compounds
- ↑ Bufadienolides at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Cardenolides at the US National Library of Medicine Medical Subject Headings (MeSH)