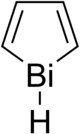Bismole
Not to be confused with Bismoll or Pepto-Bismol.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1H-Bismole | |||
| Identifiers | |||
| 89067-15-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 24751865 | ||
| |||
| |||
| Properties | |||
| C4H5Bi | |||
| Molar mass | 262.06 g·mol−1 | ||
| Related compounds | |||
| Related compounds |
Pyrrole, phosphole, arsole, stibole | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Bismole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4BiH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with bismuth replacing the nitrogen atom of pyrrole. The unsubstituted compound has not been isolated due to the high energy of the Bi-H bond. Substituted derivatives, which have been synthesized, are called bismoles.[1]
Reactions
2,5-Bis(trimethylsilyl)-3,4-dimethyl-1-phenyl-1H-bismole, for example, can be formed by the reaction of (1Z,3Z)-1,4-bis(trimethylsilyl)-1,4-diiodobuta-2,3-dimethyl-1,3-diene and diiodophenylbismuthine. Bismoles can be used to form ferrocene-like sandwich compounds.[2]
See also
References
- ↑ Suzuki, Hitomi; Komatsu, Naoki; Ogawa, Takuji; Murafuji, Toshihiro; Ikegami, Tohru; Matano, Yoshihiro (2001), "4: Bismuth-Containing Heterocycle", Organobismuth Chemistry, Elsevier, pp. 329–344, ISBN 978-0-444-20528-5
- ↑ Katritzky, Alan R.; Kirby, Gordon W.; Meth-Cohn, Otto; Rees, Charles W., eds. (1995), Synthesis: Carbon with Two Heteroatoms, Each Attached by a Single Bond, Volume 4 of Comprehensive Organic Functional Group Transformations, Elsevier, pp. 1038–1040, ISBN 978-0-08-042325-8
This article is issued from Wikipedia - version of the 9/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.