Bird vocalization

Bird vocalization includes both bird calls and bird songs. In non-technical use, bird songs are the bird sounds that are melodious to the human ear. In ornithology and birding, (relatively complex) songs are distinguished by function from (relatively simple) calls.
Definition

The distinction between songs and calls is based upon complexity, length, and context. Songs are longer and more complex and are associated with courtship and mating, while calls tend to serve such functions as alarms or keeping members of a flock in contact.[3] Other authorities such as Howell and Webb (1995) make the distinction based on function, so that short vocalizations, such as those of pigeons, and even non-vocal sounds, such as the drumming of woodpeckers and the "winnowing" of snipes' wings in display flight, are considered songs.[4] Still others require song to have syllabic diversity and temporal regularity akin to the repetitive and transformative patterns that define music. It is generally agreed upon in birding and ornithology which sounds are songs and which are calls, and a good field guide will differentiate between the two.
Bird song is best developed in the order Passeriformes. Some groups are nearly voiceless, producing only percussive and rhythmic sounds, such as the storks, which clatter their bills. In some manakins (Pipridae), the males have evolved several mechanisms for mechanical sound production, including mechanisms for stridulation not unlike those found in some insects.[5]
Song is usually delivered from prominent perches, although some species may sing when flying. The production of sounds by mechanical means as opposed to the use of the syrinx has been termed variously instrumental music by Charles Darwin, mechanical sounds[6] and more recently sonation.[7] The term sonate has been defined as the act of producing non-vocal sounds that are intentionally modulated communicative signals, produced using non-syringeal structures such as the bill, wings, tail, feet and body feathers.[7]
In extratropical Eurasia and the Americas almost all song is produced by male birds; however in the tropics and to a greater extent the desert belts of Australia and Africa it is more typical for females to sing as much as males. These differences have been known for a long time[8][9] and are generally attributed to the much less regular and seasonal climate of Australian and African arid zones requiring that birds breed at any time when conditions are favourable, although they cannot breed in many years because food supply never increases above a minimal level.[8] With aseasonal irregular breeding, both sexes must be brought into breeding condition and vocalisation, especially duetting, serves this purpose. The high frequency of female vocalisations in the tropics, Australia and Southern Africa may also relate to very low mortality rates producing much stronger pair-bonding and territoriality.[10]
Anatomy and physiology
The avian vocal organ is called the syrinx;[11] it is a bony structure at the bottom of the trachea (unlike the larynx at the top of the mammalian trachea). The syrinx and sometimes a surrounding air sac resonate to sound waves that are made by membranes past which the bird forces air. The bird controls the pitch by changing the tension on the membranes and controls both pitch and volume by changing the force of exhalation. It can control the two sides of the trachea independently, which is how some species can produce two notes at once.
Function

Scientists hypothesize that bird song has evolved through sexual selection, and experiments suggest that the quality of bird song may be a good indicator of fitness.[12] Experiments also suggest that parasites and diseases may directly affect song characteristics such as song rate, which thereby act as reliable indicators of health.[13][14] The song repertoire also appears to indicate fitness in some species.[15][16] The ability of male birds to hold and advertise territories using song also demonstrates their fitness.
Communication through bird calls can be between individuals of the same species or even across species. Birds communicate alarm through vocalizations and movements that are specific to the threat, and bird alarms can be understood by other animal species, including other birds, in order to identify and protect against the specific threat.[17] Mobbing calls are used to recruit individuals in an area where an owl or other predator may be present. These calls are characterized by wide-frequency spectra, sharp onset and termination, and repetitiveness that are common across species and are believed to be helpful to other potential "mobbers" by being easy to locate. The alarm calls of most species, on the other hand, are characteristically high-pitched, making the caller difficult to locate.[18]
Individual birds may be sensitive enough to identify each other through their calls. Many birds that nest in colonies can locate their chicks using their calls.[19] Calls are sometimes distinctive enough for individual identification even by human researchers in ecological studies.[20]
Many birds engage in duet calls. In some cases, the duets are so perfectly timed as to appear almost as one call. This kind of calling is termed antiphonal duetting.[21] Such duetting is noted in a wide range of families including quails,[22] bushshrikes,[23] babblers such as the scimitar babblers, some owls[24] and parrots.[25] In territorial songbirds, birds are more likely to countersing when they have been aroused by simulated intrusion into their territory.[26] This implies a role in intraspecies aggressive competition.
Sometimes, songs vocalized in post-breeding season act as a cue to conspecific eavesdroppers.[27] In black-throated blue warblers, males that have bred and reproduced successfully sing to their offspring to influence their vocal development, while males that have failed to reproduce usually abandon the nests and stay silent. The post-breeding song therefore inadvertently informs the unsuccessful males of particular habitats that have a higher likelihood of reproductive success. The social communication by vocalization provides a shortcut to locating high quality habitats and saves the trouble of directly assessing various vegetation structures.
Some birds are excellent vocal mimics. In some tropical species, mimics such as the drongos may have a role in the formation of mixed-species foraging flocks.[28] Vocal mimicry can include conspecifics, other species or even man-made sounds. Many hypotheses have been made on the functions of vocal mimicry including suggestions that they may be involved in sexual selection by acting as an indicator of fitness, help brood parasites, or protect against predation, but strong support is lacking for any function.[29] Many birds, especially those that nest in cavities, are known to produce a snakelike hissing sound that may help deter predators at close range.[30]
Some cave-dwelling species, including the oilbird[31] and swiftlets (Collocalia and Aerodramus spp.),[32] use audible sound (with the majority of sonic location occurring between 2 and 5 kHz[33]) to echolocate in the darkness of caves. The only bird known to make use of infrasound (at about 20 Hz) is the western capercaillie.[34]
The hearing range of birds is from below 50 Hz (infrasound) to around 12 kHz, with maximum sensitivity between 1 and 5 kHz.[16][35]
The range of frequencies at which birds call in an environment varies with the quality of habitat and the ambient sounds. The acoustic adaptation hypothesis predicts that narrow bandwidths, low frequencies, and long elements and inter-element intervals should be found in habitats with complex vegetation structures (which would absorb and muffle sounds), while high frequencies, broad bandwidth, high-frequency modulations (trills), and short elements and inter-elements may be expected in open habitats, without obstructive vegetation.[36][37][38] Low frequency songs are optimal for obstructed, densely vegetated habitats because low frequency, slowly modulated song elements are less susceptible to signal degradation by means of reverberations off of sound-reflecting vegetation. High frequency calls with rapid modulations are optimal for open habitats because they degrade less across open space.[39][40] The acoustic adaptation hypothesis also states that song characteristics may take advantage of beneficial acoustic properties of the environment. Narrow-frequency bandwidth notes are increased in volume and length by reverberations in densely vegetated habitats.[41]
It has been hypothesized that the available frequency range is partitioned, and birds call so that overlap between different species in frequency and time is reduced. This idea has been termed the "acoustic niche".[42] Birds sing louder and at a higher pitch in urban areas, where there is ambient low-frequency noise.[43][44] Traffic noise was found to decrease reproductive success in the great tit (Parsus major) due to the overlap in acoustic frequency.[45] An increase in song volume restored fitness to birds in urban areas, as did higher frequency songs.[46]
Learning

The songs of different species of birds vary and are generally typical of the species. Species vary greatly in the complexity of their songs and in the number of distinct kinds of song they sing (up to 3000 in the brown thrasher); individuals within some species vary in the same way. In a few species, such as lyrebirds and mockingbirds, songs imbed arbitrary elements learned in the individual's lifetime, a form of mimicry (though maybe better called "appropriation" [Ehrlich et al.], as the bird does not pass for another species). As early as 1773, it was established that birds learned calls, and cross-fostering experiments succeeded in making linnet Acanthis cannabina learn the song of a skylark, Alauda arvensis.[48] In many species, it appears that although the basic song is the same for all members of the species, young birds learn some details of their songs from their fathers, and these variations build up over generations to form dialects.[49]
Song learning in juvenile birds occurs in two stages: sensory learning, which involves the juvenile listening to the father or other conspecific bird and memorizing the spectral and temporal qualities of the song (song template), and sensorimotor learning, which involves the juvenile bird producing its own vocalizations and practicing its song until it accurately matches the memorized song template.[50] During the sensorimotor learning phase, song production begins with highly variable sub-vocalizations called "sub-song", which is akin to babbling in human infants.[51] Soon after, the juvenile song shows certain recognizable characteristics of the imitated adult song, but still lacks the stereotypy of the crystallized song – this is called "plastic song".[52] Finally, after two or three months of song learning and rehearsal (depending on species), the juvenile produces a crystallized song, characterized by spectral and temporal stereotypy (very low variability in syllable production and syllable order).[53] Some birds, such as zebra finches, which are the most popular species for birdsong research, have overlapping sensory and sensorimotor learning stages.[47]
Research has indicated that birds' acquisition of song is a form of motor learning that involves regions of the basal ganglia. Further, the PDP (see Neuroanatomy below) has been considered homologous to a mammalian motor pathway originating in the cerebral cortex and descending through the brain stem, while the AFP has been considered homologous to the mammalian cortical pathway through the basal ganglia and thalamus.[52] Models of bird-song motor learning can be useful in developing models for how humans learn speech.[54] In some species such as zebra finches, learning of song is limited to the first year; they are termed "age-limited" or "close-ended" learners. Other species such as the canaries can develop new songs even as sexually mature adults; these are termed "open-ended" learners.[55][56]
Researchers have hypothesized that learned songs allow the development of more complex songs through cultural interaction, thus allowing intraspecies dialects that help birds to identify kin and to adapt their songs to different acoustic environments.[57]
Neuroanatomy
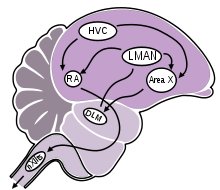
The acquisition and learning of bird song involves a group of distinct brain areas that are aligned in two connecting pathways:[52]
- Anterior forebrain pathway (vocal learning): composed of Area X, which is a homologue to mammalian basal ganglia; the lateral part of the magnocellular nucleus of anterior nidopallium (LMAN), also considered a part of the avian basal ganglia; and the dorso-lateral division of the medial thalamus (DLM).
- Posterior descending pathway (vocal production): composed of HVC (proper name, although sometimes referred to as the high vocal center); the robust nucleus of the arcopallium (RA); and the tracheosyringeal part of the hypoglossal nucleus (nXIIts).[58][59]
The posterior descending pathway (PDP) is required throughout a bird's life for normal song production, while the anterior forebrain pathway (AFP) is necessary for song learning in juvenilles and plasticity/maintenance in adults, but not for adult song production.[60]
Both neural pathways in the song system begin at the level of HVC, which projects information both to the RA (premotor nucleus) and to Area X of the anterior forebrain. Information in the posterior descending pathway (also referred to as the vocal production or motor pathway) descends from HVC to RA, and then from RA to the tracheosyringeal part of the hypoglossal nerve (nXIIts), which then controls muscular contractions of the syrinx.[52][61]
Information in the anterior forebrain pathway is projected from HVC to Area X (basal ganglia), then from Area X to the DLM (thalamus), and from DLM to LMAN, which then links the vocal learning and vocal production pathways through connections back to the RA. Some investigators have posited a model in which the connection between LMAN and RA carries an instructive signal based on evaluation of auditory feedback (comparing the bird's own song to the memorized song template), which adaptively alters the motor program for song output.[60][62] The generation of this instructive signal could be facilitated by auditory neurons in Area X and LMAN that show selectivity for the temporal qualities of the bird's own song (BOS) and its tutor song, providing a platform for comparing the BOS and the memorized tutor song.[62][63]
Models regarding the real-time error-correction interactions between the AFP and PDP will be considered in the future. Other current research has begun to explore the cellular mechanisms underlying HVC control of temporal patterns of song structure and RA control of syllable production.[64] Brain structures involved in both pathways show sexual dimorphism in many bird species, usually causing males and females to sing differently. Some of the known types of dimorphisms in the brain include the size of nuclei, the number of neurons present, and the number of neurons connecting one nucleus to another.[65] In the extremely dimorphic zebra finches (Taeniopygia guttata), a species in which only males typically sing, the size of the HVC and RA are approximately three to six times larger in males than in females, and Area X does not appear to be recognizable in females.[66] Research suggests that exposure to sex steroids during early development is partially responsible for these differences in the brain. Female zebra finches treated with estradiol after hatching followed by testosterone or dihydrotestosterone (DHT) treatment in adulthood will develop an RA and HVC similar in size to males and will also display male-like singing behavior.[67] Hormone treatment alone does not seem to produce female finches with brain structures or behavior exactly like males. Furthermore, other research has shown results that contradict what would be expected based on our current knowledge of mammalian sexual differentiation. For example, male zebra finches castrated or given sex steroid inhibitors as hatchlings still develop normal masculine singing behavior.[65] This suggests that other factors, such as the activation of genes on the z chromosome, might also play a role in normal male song development.[68]
Hormones also have activational effects on singing and the song nuclei in adult birds. In canaries (Serinus canaria), females normally sing less often and with less complexity than males. However, when adult females are given androgen injections, their singing will increase to an almost male-like frequency.[69] Furthermore, adult females injected with androgens also show an increased size in the HVC and RA regions.[70] Melatonin is another hormone that is also believed to influence song behavior in adults, as many songbirds show melatonin receptors in neurons of the song nuclei.[71] Both the European starling (Sturnus vulgaris) and house sparrow (Passer domesticus) have demonstrated changes in song nuclei correlated with differing exposures to darkness and secretions of melatonin.[72][73] This suggests that melatonin might play a role in the seasonal changes of singing behavior in songbirds that live in areas where the amount of daylight varies significantly throughout the year. Several other studies have looked at seasonal changes in the morphology of brain structures within the song system and have found that these changes (adult neurogenesis, gene expression) are dictated by photoperiod, hormonal changes and behavior.[74][75]
The gene FOXP2, defects of which affect both speech production and comprehension of language in humans, becomes highly expressed in Area X during periods of vocal plasticity in both juvenile zebra finches and adult canaries.[76]
Auditory feedback in birdsong learning
Early experiments by Thorpe in 1954 showed the importance of a bird being able to hear a tutor's song. When birds are raised in isolation, away from the influence of conspecific males, they still sing. While the song they produce, called "isolate song", resembles the song of a wild bird, it shows distinctly different characteristics from the wild song and lacks its complexity.[77] The importance of the bird being able to hear itself sing in the sensorimotor period was later discovered by Konishi. Birds deafened before the song-crystallization period went on to produce songs that were distinctly different from the wild type and isolate song.[78][79] Since the emergence of these findings, investigators have been searching for the neural pathways that facilitate sensory/sensorimotor learning and mediating the matching of the bird's own song with the memorized song template.
Several studies over recent decades have looked at the neural mechanisms underlying birdsong learning by performing lesions to relevant brain structures involved in the production or maintenance of song or by deafening birds before and/or after song crystallization. Another recent experimental approach was recording the bird's song and then playing it back while the bird is singing, causing perturbed auditory feedback (the bird hears the superposition of its own song and a fragmented portion of a previous song syllable).[53] After Nordeen & Nordeen[80] made a landmark discovery as they demonstrated that auditory feedback was necessary for the maintenance of song in adult birds with crystallized song, Leonardo & Konishi (1999) designed an auditory feedback perturbation protocol in order to explore the role of auditory feedback in adult song maintenance further, to investigate how adult songs deteriorate after extended exposure to perturbed auditory feedback, and to examine the degree to which adult birds could recover crystallized song over time after being removed from perturbed feedback exposure. This study offered further support for role of auditory feedback in maintaining adult song stability and demonstrated how adult maintenance of crystallized birdsong is dynamic rather than static.
Brainard & Doupe (2000) posit a model in which LMAN (of the anterior forebrain) plays a primary role in error correction, as it detects differences between the song produced by the bird and its memorized song template and then sends an instructive error signal to structures in the vocal production pathway in order to correct or modify the motor program for song production. In their study, Brainard & Doupe (2000) showed that while deafening adult birds led to the loss of song stereotypy due to altered auditory feedback and non-adaptive modification of the motor program, lesioning LMAN in the anterior forebrain pathway of adult birds that had been deafened led to the stabilization of song (LMAN lesions in deafened birds prevented any further deterioration in syllable production and song structure).
Currently, there are two competing models that elucidate the role of LMAN in generating an instructive error signal and projecting it to the motor production pathway:
- Bird’s Own Song (BOS)-tuned Error Correction Model
- During singing, the activation of LMAN neurons will depend on the match between auditory feedback from the song produced by the bird and the stored song template. If this is true, then the firing rates of LMAN neurons will be sensitive to changes in auditory feedback.
- Efference Copy Model of Error Correction
- An efference copy of the motor command for song production is the basis of the real-time error-correction signal. During singing, activation of LMAN neurons will depend on the motor signal used to generate the song, and the learned prediction of expected auditory feedback based on that motor command. Error correction would occur more rapidly in this model.
Leonardo [81] tested these models directly by recording spike rates in single LMAN neurons of adult zebra finches during singing in conditions with normal and perturbed auditory feedback. His results did not support the BOS-tuned error correction model, as the firing rates of LMAN neurons were unaffected by changes in auditory feedback and therefore, the error signal generated by LMAN appeared unrelated to auditory feedback. Moreover, the results from this study supported the predictions of the efference copy model, in which LMAN neurons are activated during singing by the efference copy of the motor signal (and its predictions of expected auditory feedback), allowing the neurons to be more precisely time-locked to changes in auditory feedback.
Mirror neurons and vocal learning
A mirror neuron is a neuron that discharges both when an individual performs an action and when he/she perceives that same action being performed by another.[82] These neurons were first discovered in macaque monkeys, but recent research suggests that mirror neuron systems may be present in other animals including humans.[83]
Mirror neurons have the following characteristics:[82]
- They are located in the premotor cortex.
- They exhibit both sensory and motor properties.
- They are action-specific – mirror neurons are only active when an individual is performing or observing a certain type of action (e.g., grasping an object).
Because mirror neurons exhibit both sensory and motor activity, some researchers have suggested that mirror neurons may serve to map sensory experience onto motor structures.[84] This has implications for birdsong learning– many birds rely on auditory feedback to acquire and maintain their songs. Mirror neurons may be mediating this comparison of what the bird hears, how it compares to a memorized song template, and what he produces.

In search of these auditory-motor neurons, Jonathan Prather and other researchers at Duke University recorded the activity of single neurons in the HVCs of swamp sparrows.[85] They discovered that the neurons that project from the HVC to Area X (HVCX neurons) are highly responsive when the bird is hearing a playback of his own song. These neurons also fire in similar patterns when the bird is singing that same song. Swamp sparrows employ 3-5 different song types, and the neural activity differs depending on which song is heard or sung. The HVCX neurons only fire in response to the presentation (or singing) of one of the songs, the primary song type. They are also temporally selective, firing at a precise phase in the song syllable.
Prather, et al. found that during the short period of time before and after the bird sings, his HVCX neurons become insensitive to auditory input. In other words, the bird becomes "deaf" to his own song. This suggests that these neurons are producing a corollary discharge, which would allow for direct comparison of motor output and auditory input.[86] This may be the mechanism underlying learning via auditory feedback. These findings are also in line with Leonardo's (2004) efference copy model of error correction in birdsong learning and production.
Overall, the HVCX auditory motor neurons in swamp sparrows are very similar to the visual motor mirror neurons discovered in primates. Like mirror neurons, the HVCX neurons:
- Are located in a premotor brain area
- Exhibit both sensory and motor properties
- Are action-specific – a response is only triggered by the "primary song type"
The function of the mirror neuron system is still unclear. Some scientists speculate that mirror neurons may play a role in understanding the actions of others, imitation, theory of mind and language acquisition, though there is currently insufficient neurophysiological evidence in support of these theories.[84] Specifically regarding birds, it is possible that the mirror neuron system serves as a general mechanism underlying vocal learning, but further research is needed. In addition to the implications for song learning, the mirror neuron system could also play a role in territorial behaviors such as song-type matching and countersinging.[87][88]
Identification and systematics
The specificity of bird calls has been used extensively for species identification. The calls of birds have been described using words or nonsense syllables or line diagrams.[89] Common terms in English include words such as quack, chirp and chirrup. These are subject to imagination and vary greatly; a well-known example is the white-throated sparrow's song, given in Canada as O sweet Canada Canada Canada and in New England as Old Sam Peabody Peabody Peabody (also Where are you Frederick Frederick Frederick?). In addition to nonsense words, grammatically correct phrases have been constructed as likenesses of the vocalizations of birds. For example, the barred owl produces a motif which some bird guides describe as Who cooks for you? Who cooks for you all? with the emphasis placed on you.[90]

The use of spectrograms to visualize bird song was first introduced by W. H. Thorpe.[91][92] These visual representations are also called sonograms or sonagrams. Beginning in 1983, some field guides for birds use sonograms to document the calls and songs of birds.[93] The sonogram is objective, unlike descriptive phrases, but proper interpretation requires experience. Sonograms can also be roughly converted back into sound.[94][95]
Bird song is an integral part of bird courtship and is a pre-zygotic isolation mechanism involved in the process of speciation. Many allopatric sub-species show differences in calls. These differences are sometimes minute, often detectable only in the sonograms. Song differences in addition to other taxonomic attributes have been used in the identification of new species.[96] The use of calls has led to proposals for splitting of species complexes such as those of the Mirafra bushlarks.[97]
Smartphone apps like Bird Song Id USA[98] or Merlin Bird ID[99] enable automatic recognition of bird using sounds.[100] Like Shazam, those apps works by recording nearby noises on smartphones to analyze it in real-time and identify the bird species by their songs and call.
Bird recognition apps rely on spectrographic audio analysis: it records several seconds of the bird singing, finds distinguishing aspects of a bird’s song, and searches the database for matches.
Recording
The first known recording of birdsong was made in 1889 by Ludwig Koch,[101] who went on to become an eminent wildlife recordist and BBC natural history presenter.[101]
Other notable birdsong recordists include Eric Simms, Chris Watson and, in France, Jean-Claude Roché, François Charron, Fernand Deroussen.
Bird language
The language of the birds has long been a topic for anecdote and speculation. That calls have meanings that are interpreted by their listeners has been well demonstrated. Domestic chickens have distinctive alarm calls for aerial and ground predators, and they respond to these alarm calls appropriately.[102][103] However, a language has, in addition to words, grammar (that is, structures and rules). Studies to demonstrate the existence of language have been difficult due to the range of possible interpretations. Research on parrots by Irene Pepperberg is claimed to demonstrate the innate ability for grammatical structures, including the existence of concepts such as nouns, adjectives and verbs.[104] Studies on starling vocalizations have also suggested that they may have recursive structures.[105]
The term bird language may also more informally refer to patterns in bird vocalizations that communicate information to other birds or other animals in general.[106]
Bird song and music
In music, birdsong has influenced composers and musicians in several ways: they can be inspired by birdsong; they can intentionally imitate bird song in a composition, as Vivaldi and Beethoven did, along with many later composers; they can incorporate recordings of birds into their works, as Ottorino Respighi first did; or like Beatrice Harrison and David Rothenberg, they can duet with birds.[107][108][109] Authors including Rothenberg have claimed that birds sing on traditional scales as used in human music,[110][111][112] but at least one songbird does not choose notes in this way.[113] However, among birds which habitually borrow phrases or sounds from other species, the way they use variations of rhythm, relationships of musical pitch, and combinations of notes can resemble music.[114] The similar motor constraints on human and avian song may have driven these to have similar song structures, including "arch-shaped and descending melodic contours in musical phrases", long notes at the ends of phrases, and typically small differences in pitch between adjacent notes, at least in birds with a strong song structure like the Eurasian treecreeper Certhia familiaris.[115]
Bird song and poetry
Bird song is a popular subject in poetry. Famous examples inspired by bird song include the 1177 Persian poem "The Conference of the Birds", in which the birds of the world assemble under the wisest bird, the hoopoe, to decide who is to be their king.[116] In English poetry, John Keats's 1819 "Ode to a Nightingale" and Percy Bysshe Shelley's 1820 "To a Skylark" are popular classics.[117][118] Ted Hughes's 1970 collection of poems about a bird character, "Crow", is considered one of his most important works.[119] Bird poems by Gerard Manley Hopkins include "Sea and Skylark" and "The Windhover".[120]
See also
References
- ↑ Sclater, PL (1860). "List of Birds collected by Mr. Fraser in Ecuador, at Nanegal, Calacali, Perucho, and Puellaro, with notes and descriptions of new species". Proc. Zool. Soc. London: 83–97.
- ↑ Darwin, Charles (1871). The descent of man and selection in relation to sex. volume 2. John Murray, London. pp. 65–66. ISBN 1-108-00510-1.
- ↑ Ehrlich, Paul R.; David S. Dobkin & Darryl Wheye. ""Bird Voices" and "Vocal Development" from Birds of Stanford essays". Retrieved 9 Sep 2008.
- ↑ Howell, Steve N. G. & Sophie Webb (1995). A Guide to the Birds of Mexico and Northern Central America. Oxford University Press. ISBN 0-19-854012-4.
- ↑ Bostwick, Kimberly S. & Richard O. Prum (2005). "Courting Bird Sings with Stridulating Wing Feathers". Science. 309 (5735): 736. doi:10.1126/science.1111701. PMID 16051789.
- ↑ Manson-Barr, P. and Pye, J. D. (1985). Mechanical sounds. In A Dictionary of Birds (ed. B. Campbell and E. Lack), pp. 342-344. Staffordshire: Poyser.
- 1 2 Bostwick, Kimberly S. & Richard O. Prum (2003). "High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves)". The Journal of Experimental Biology. 206 (Pt 20): 3693–3706. doi:10.1242/jeb.00598. PMID 12966061.
- 1 2 Robinson, Angus; “The Biological Significance of Bird Song in Australia” in Emu; 48(4), pp. 291-315
- ↑ Hartshorne, Charles; “Some Biological Principles Applicable to Song Behavior” in The Wilson Bulletin Vol. 70, No. 1 (Mar., 1958), pp. 41-56
- ↑ Slater, Peter J. B. and Mann, Nigel I.; “Why do the females of many bird species sing in the tropics?”; in Journal of Avian Biology Volume 35, Issue 4, pages 289–294, July 2004
- ↑ Attenborough, D. 1998. The Life of Birds. BBC publication.0563-38792-0
- ↑ Read, A. W. & D. M. Weary (1990). "Sexual selection and the evolution of bird song: A test of the Hamilton-Zuk hypothesis". Behavioral Ecology and Sociobiology. 26 (1): 47–56. doi:10.1007/BF00174024.
- ↑ Garamszegi, L. Z.; A. P. Møller; János Török; Gábor Michl; Péter Péczely; Murielle Richard (2004). "Immune challenge mediates vocal communication in a passerine bird: an experiment". Behavioral Ecology. 15 (1): 148–157. doi:10.1093/beheco/arg108.
- ↑ Redpath, S. M.; Bridget M Appleby; Steve J Petty (2000). "Do male hoots betray parasite loads in Tawny Owls?". Journal of Avian Biology. 31 (4): 457–462. doi:10.1034/j.1600-048X.2000.310404.x.
- ↑ Reid, J. M., Peter Arcese, Alice L. E. V. Cassidy, Sara M. Hiebert, James N. M. Smith, Philip K. Stoddard, Amy B. Marr, and Lukas F. Keller (2005). "Fitness Correlates of Song Repertoire Size in Free-Living Song Sparrows (Melospiza melodia)". The American Naturalist. 165 (3): 299–310. doi:10.1086/428299. PMID 15729661.
- 1 2 Møller AP; J. Erritzøe; L. Z. Garamszegi (2005). "Covariation between brain size and immunity in birds: implications for brain size evolution" (PDF). Journal of Evolutionary Biology. 18 (1): 223–237. doi:10.1111/j.1420-9101.2004.00805.x. PMID 15669979.
- ↑ "A Bird's World: Speaking in a Bird's Language". Museum of Science, Boston. 2008.
- ↑ Marler, P. (1955). "Characteristics of some animal calls". Nature. 176 (4470): 6–8. doi:10.1038/176006a0.
- ↑ Lengagne, T., J. Lauga and T. Aubin (2001). "Intra-syllabic acoustic signatures used by the King Penguin in parent-chick recognition: an experimental approach" (PDF). The Journal of Experimental Biology. 204 (Pt 4): 663–672. PMID 11171348.
- ↑ Wayne Delport; Alan C Kemp; J. Willem H Ferguson (2002). "Vocal identification of individual African Wood Owls Strix woodfordii: a technique to monitor long-term adult turnover and residency". Ibis. 144 (1): 30–39. doi:10.1046/j.0019-1019.2001.00019.x.
- ↑ Thorpe, W. H. (23 February 1963). "Antiphonal Singing in Birds as Evidence for Avian Auditory Reaction Time". Nature. 197 (4869): 774–776. doi:10.1038/197774a0.
- ↑ Stokes, A. W.; H. W. Williams (1968). "Antiphonal calling in quail" (PDF). Auk. 85: 83–89. doi:10.2307/4083626.
- ↑ Harris, Tony; Franklin, Kim (2000). Shrikes and Bush-Shrikes. Princeton University Press. pp. 257–260. ISBN 0-691-07036-9.
- ↑ Osmaston, B. B. (1941). ""Duetting" in birds". Ibis. 5 (2): 310–311. doi:10.1111/j.1474-919X.1941.tb00620.x.
- ↑ Power, D. M. (1966). "Antiphonal duetting and evidence for auditory reaction time in the Orange-chinned Parakeet". Auk. 83: 314–319. doi:10.2307/4083033.
- ↑ Hyman, Jeremy (2003). "Countersinging as a signal of aggression in a territorial songbird" (PDF). Animal Behaviour. 65 (6): 1179–1185. doi:10.1006/anbe.2003.2175.
- ↑ Betts, M.G.; Hadley, A.S.; Rodenhouse, N.; Nocera, J.J. (2008). "Social Information Trumps Vegetation Structure in Breeding-Site Selection by a Migrant Songbird". Proceedings: Biological Sciences. 1648. 275: 2257–2263. doi:10.1098/rspb.2008.0217.
- ↑ Goodale, E. & Kotagama, S. W. (2005). "Testing the roles of species in mixed-species bird flocks of a Sri Lankan rain forest". Journal of Tropical Ecology. 21 (6): 669–676. doi:10.1017/S0266467405002609.
- ↑ Kelley, LA; RL Coe; JR Madden; SD Healy (2008). "Vocal mimicry in songbirds". Animal Behaviour. 76 (3): 521–528. doi:10.1016/j.anbehav.2008.04.012.
- ↑ Marler, Peter; Hans Willem Slabbekoorn (2004). Nature's music: the science of birdsong. Academic Press. p. 145. ISBN 0-12-473070-1.
- ↑ Suthers RA & Hector DH (1985). "The physiology of vocalization by the echolocating Oilbird, Steatornis caripensis". J. Comp. Physiol. 156 (2): 243–266. doi:10.1007/BF00610867.
- ↑ Suthers RA & Hector DH (1982). "Mechanism for the production of echolocating clicks by the Grey Swiftlet, Collocalia spodiopygia". J. Comp. Physiol. A. 148 (4): 457–470. doi:10.1007/BF00619784.
- ↑ Coles RB, Konishi M, Pettigrew JD (1987). "Hearing and echolocation in the Australian Grey Swiftlet, Collocalia spodiopygia". J. Exp. Biol. 129: 365–371.
- ↑ Lieser M, P. Berthold1 and G. A. Manley (2005). "Infrasound in the capercaillie ( Tetrao urogallus )". Journal of Ornithology. 146 (4): 395–398. doi:10.1007/s10336-005-0003-y.
- ↑ Dooling, R.J. (1982). Auditory perception in birds. Acoustic Communication in Birds, Vol. 1 (eds D.E. Kroodsma & E.H. Miller). pp. 95–130.
- ↑ Derryberry, Elizabeth (July 2009). "Ecology Shapes Birdsong Evolution: Variation in Morphology and Habitat Explains Variation in White-Crowned Sparrow Song". The American Naturalist. 174 (1): 24–33. doi:10.1086/599298.
- ↑ Boncoraglio, G. & Nicola Saino (2007). "Habitat structure and the evolution of bird song: a meta-analysis of the evidence for the acoustic adaptation hypothesis". Functional Ecology. 21: 134–142. doi:10.1111/j.1365-2435.2006.01207.x.
- ↑ Morton, E.S. (1975). "Ecological sources of selection on avian sounds". American Naturalist. 109 (965): 17–34. doi:10.1086/282971.
- ↑ Ey, Elodie; Fischer, J. (13 April 2012). "The "acoustic adaptation hypothesis" - a review of the evidence from birds, anurans and mammals". Bioacoustics. 19 (1-2): 21–48.
- ↑ Tubaro, Pablo L.; Segura, Enrique T. (November 1994). "Dialect Differences in the Song of Zonotrichia capensis in the Southern Pampas: A Test of the Acoustic Adaptation Hypothesis". The Condor. 96 (4): 1084–1088. doi:10.2307/1369117.
- ↑ Slabbekoorn, Hans; Ellers, Jacintha; Smith, Thomas B. (2002). "Birdsong and sound transmission: the benefits of reverberations". The Condor. 104: 564–573. doi:10.1650/0010-5422(2002)104[0564:basttb]2.0.co;2.
- ↑ Krause, Bernard L. (1993). "The Niche Hypothesis" (PDF). The Soundscape Newsletter. 06.
- ↑ Henrik Brumm (2004). "The impact of environmental noise on song amplitude in a territorial bird". Journal of Animal Ecology. 73 (3): 434–440. doi:10.1111/j.0021-8790.2004.00814.x.
- ↑ Slabbekoorn, H. & Peet, M. (2003). "Birds sing at a higher pitch in urban noise". Nature. 424 (6946): 267. doi:10.1038/424267a. PMID 12867967.
- ↑ Halfwerk, Wouter; Holleman, L.J.M.; Lessells, C.M.; Slabbekoorn, H. (February 2011). "Negative impact of traffic nosie on avian reproductive success.". Journal of Applied Ecology. 48 (1): 210–219. doi:10.1111/j.1365-2664.2010.01914.x.
- ↑ Luther, David A.; Derryberry, E.P. (April 2012). "Birdsongs keep pace with city life: changes in song over time in an urban songbird affects communication". Animal Behaviour. 83 (4): 1059–1066. doi:10.1016/j.anbehav.2012.01.034.
- 1 2 Brainard, M. S. & Doupe, A. J. (2002). "What songbirds teach us about learning". Nature. 417 (6886): 351–358. doi:10.1038/417351a. PMID 12015616.
- ↑ Barrington, D. (1773). "Experiments and observations on the singing of birds". Philosophical Transactions of the Royal Society. 63: 249–291. doi:10.1098/rstl.1773.0031.
- ↑ Marler, P.; M. Tamura (1962). "Song dialects in three populations of the white-crowned sparrow". Condor. 64 (5): 368–377. doi:10.2307/1365545. JSTOR 1365545.
- ↑ Konishi, M. (2010). "From central pattern generator to sensory template in the evolution of birdsong". Brain & Language. 15: 18–20. doi:10.1016/j.bandl.2010.05.001. PMID 20955898.
- ↑ Naguib, M. and K. Riebel. (2014). Singing in Space and Time: The Biology of Birdsong. In: Witzany, G. (ed). Biocommunication of Animals. Springer. 233-247. ISBN 978-94-007-7413-1.
- 1 2 3 4 Nottebohm,F. (2005). "The Neural Basis of Birdsong". PLoS Biol. 3 (5): 163. doi:10.1371/journal.pbio.0030164. PMC 1110917
 . PMID 15884976.
. PMID 15884976. - 1 2 Leonardo, A.; Konishi, M. (1999). "Decrystallization of adult birdsong by perturbation of auditory feedback". Nature. 399 (6735): 466–470. doi:10.1038/20933. PMID 10365958.
- ↑ Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA (2004). "Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction". J. Neurosci. 24 (13): 3152–63. doi:10.1523/JNEUROSCI.5589-03.2004. PMID 15056695.
- ↑ Nottebohm, F. (2004). "The road we travelled: discovery, choreography, and significance of brain replaceable neurons". Annals of the New York Academy of Sciences. 1016: 628–658. doi:10.1196/annals.1298.027. PMID 15313798.
- ↑ Brenowitz, Eliot A. & Michael D. Beecher (2005). "Song learning in birds: diversity and plasticity, opportunities and challenges" (PDF). Trends in Neurosciences. 28 (3): 127–132. doi:10.1016/j.tins.2005.01.004. PMID 15749165.
- ↑ Slater, P. J. B. (1989). "Bird song learning: causes and consequences". Ethol. Ecol. Evol. 1: 19–46. doi:10.1080/08927014.1989.9525529.
- ↑ Brainard, M. S. & Doupe, A. J. (2000). "Auditory feedback in learning and maintenance of vocal behavior". Nature Reviews Neuroscience. 1 (1): 31–40. doi:10.1038/35036205. PMID 11252766.
- ↑ Carew, Thomas J. (2000). Behavioral Neurobiology: The Cellular Organization of Natural Behavior. Sinauer Associates, Inc. ISBN 978-0-87893-092-0.
- 1 2 Kao, M.H.; Doupe, A.J.; Brainard, M.S. (2005). "Contributions of an avian basal ganglia-forebrain circuit to real=time modulation of song". Nature. 433: 638–642. doi:10.1038/nature03127.
- ↑ Suthers, R. (2004). "How birds sing and why it matters". In Marler, P.; Slabbekoorn, H. Nature's music:The science of birdsong. Academic Press. pp. 272–295. ISBN 0-12-473070-1.
- 1 2 Brainard, M. S. & Doupe, A. J. (2000). "Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations". Nature. 404 (6779): 762–766. doi:10.1038/35008083. PMID 10783889.
- ↑ Kojima, S.; Doupe, A. (2008). "Neural encoding of auditory temporal context in a songbird basal ganglia nucleus, and its independence of birds' song experience". European Journal of Neuroscience. 25 (5): 1231–1244. doi:10.1111/j.1460-9568.2008.06083.x. PMC 2408885
 . PMID 18364039.
. PMID 18364039. - ↑ Long, M.A.; Jin, D.Z.; Fee, M.S. (2010). "Support for a synaptic chain model of neuronal sequence generation". Nature. 468: 394–399. doi:10.1038/nature09514.
- 1 2 Balthazart, J. and Adkins-Regan (2002). "Sexual differentiation of brain and behavior in birds". Hormones, Brain and Behavior. 4: 223–301. doi:10.1016/b978-012532104-4/50068-8.
- ↑ Nottebohm, F. & Arnold, A.P. (1976). "Sexual dimorphism in vocal control areas of the songbird brain". Science. 194 (4261): 211–213. doi:10.1126/science.959852. PMID 959852.
- ↑ Gurney, M.E. & Konishi, M. (1980). "Hormone-induced sexual differentiation of brain and behavior in zebra finches". Science. 208 (4450): 1380–1383. doi:10.1126/science.208.4450.1380.
- ↑ Tomaszycki, M.L.; Peabody, C.; Replogle, K.; Clayton, D.F; Tempelman, R.J.; Wade, J. (2009). "Sexual differentiation of the zebra finch song system: potential roles for sex chromosome genes". BMC Neuroscience. 10: 24. doi:10.1186/1471-2202-10-24.
- ↑ Leonard, S. L. (1939). "Induction of singing in female canaries by injections of male hormone". Proc. Soc. exp. Biol. 41: 429–436. doi:10.3181/00379727-41-10631.
- ↑ Nottebohm, F. (1980). "Testosterone triggers growth of brain vocal control nuclei in adult female canaries". Brain Research. 189 (2): 736. doi:10.1016/0006-8993(80)90102-X. PMID 7370785.
- ↑ Ball, G.F. & Balthazart, J. (2002). "Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds". Hormones, Brain, and Behavior. 2: 649–798. doi:10.1016/b978-012532104-4/50034-2.
- ↑ Bentley, G.E.; Van’t Hof, T.J.; Ball, G.F. (1999). "Seasonal neuroplasticity in the songbird telencephalon: A role for melatonin". Proceedings of the National Academy of Sciences of the United States of America. 96 (8): 4674–4679. doi:10.1073/pnas.96.8.4674.
- ↑ Cassone, V.M., Bartell, P.A., Earnest D.J., and Kumar, V. (2008). "Duration of melatonin regulates seasonal changes in song control nuclei of the house sparrow, Passer domesticus: Independence from gonads and circadian entrainment". Journal of Biological Rhythms. 23 (1): 49–58. doi:10.1177/0748730407311110.
- ↑ Ball, G.F.; Auger, C.J.; Bernard, D.J.; Charlier, T.D.; Sartor, J.J.; Riters, L.V.; Balthazart, J. (2004). "Seasonal plasticity in the song control system: Multiple brain sites of steroid hormone action and the importance of variation in song behavior". Annals of the New York Academy of Sciences. 1016: 586–610. doi:10.1196/annals.1298.043.
- ↑ London, S.E.; Replogle, K.; Clayton, D.F. (2009). "Developmental shifts in gene expression in the auditory forebrain during the sensitive period for song learning". Developmental Neurobiology. 69 (7): 436–450. doi:10.1002/dneu.20719. PMC 2765821
 . PMID 19360720.
. PMID 19360720. - ↑ Scharff C, Haesler S (2005). "An evolutionary perspective on FoxP2: strictly for the birds?". Current Opinion in Neurobiology. 15 (6): 694–703. doi:10.1016/j.conb.2005.10.004. PMID 16266802.
- ↑ Thorpe, W. (1954). "The process of song-learning in the chaffinch as studied by means of the sound spectrograph". Nature. 173 (4402): 465–469. doi:10.1038/173465a0.
- ↑ Konishi, M. (1965). "The role of auditory feedback on the control of vocalization in the white-crowned sparrow". Zeitschrift für Tierpsychologie. 22 (7): 770–783. PMID 5874921.
- ↑ Marler, P. (1970). "A comparative approach to vocal learning: Song development in the white-crowned sparrows". Journal of Comparative Physiological Psychology. 71: 1–25. doi:10.1037/h0029144.
- ↑ Nordeen, K.W.; Nordeen, E.J. (1994). "Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches". Behavioral and Neural Biology. 71 (1): 58–66. doi:10.1016/0163-1047(92)90757-U.
- ↑ Leonardo, A. (2004). "Experimental test of error-correction birdsong model". Proceedings of the National Academy of Sciences of the United States of America. 101 (48): 16935–16940. doi:10.1073/pnas.0407870101.
- 1 2 Rizzolatti G.; Craighero L. (2004). "The mirror-neuron system". Annu. Rev. Neurosci. 27: 169–192. doi:10.1146/annurev.neuro.27.070203.144230. PMID 15217330.
- ↑ Oberman L. M.; Pineda J. A.; Ramachandran V. S. (2007). "The human mirror neuron system: A link between action observation and social skills". Social Cognitive and Affective Neuroscience. 2 (1): 62–66. doi:10.1093/scan/nsl022. PMC 2555434
 . PMID 18985120.
. PMID 18985120. - 1 2 Dinstein I.; Thomas C.; Behrmann M.; Heeger D.J. (2008). "A mirror up to nature". Current Biology. 18: R13–18. doi:10.1016/j.cub.2007.11.004. PMC 2517574
 . PMID 18177704.
. PMID 18177704. - ↑ Prather J. F.; Peters S.; Nowicki S.; Mooney R. (2008). "Precise auditory-vocal mirroring in neurons for learned vocal communication". Nature. 451 (7176): 305–310. doi:10.1038/nature06492. PMID 18202651.
- ↑ Tchernichovski O.; Wallman J. (2008). "Behavioral neuroscience: Neurons of imitation". Nature. 451 (7176): 249–250. doi:10.1038/451249a. PMID 18202627.
- ↑ Miller G (2008). "Mirror neurons may help songbirds stay in tune". Science. 319 (5861): 269. doi:10.1126/science.319.5861.269a. PMID 18202262.
- ↑ Richard Mooney (5 June 2014). "Auditory–vocal mirroring in songbirds" (PDF). Philosophical Transactions of the Royal Society B: Biological Sciences Online. Retrieved December 16, 2015.
- ↑ Saunders, Aretas A (1951) Guide to Bird Songs. Doubleday and Company
- ↑ Sibley, David (2000). The Sibley Guide to Birds. Knopf. ISBN 0-679-45122-6.
- ↑ Thorpe, W. H. (1958). "The learning of song patterns by birds, with especial reference to the song of the chaffinch Fringilla coelebs". Ibis. 100 (4): 535–570. doi:10.1111/j.1474-919X.1958.tb07960.x.
- ↑ Slater, P. J. B. (2003). "Fifty years of bird song research: a case study in animal behaviour". Animal Behaviour. 65 (4): 633–639. doi:10.1006/anbe.2003.2051.
- ↑ Robbins, Chandler S., Bertel Bruun, Herbert S. Zim, Arthur Singer (1983). A Guide To Field Identification: Birds of North America. Golden Field Guides (Second ed.). Western Publishing Company. p. 14. ISBN 0-307-33656-5.
- ↑ Meijer, P.B.L. (1992). "An Experimental System for Auditory Image Representations". IEEE Transactions on Biomedical Engineering. 39 (2): 112–121. doi:10.1109/10.121642. PMID 1612614.
- ↑ "US Patent. 20030216649. Audible output sonogram analyzer". Freepatentsonline.com. 2003-11-20. Retrieved 2014-06-03.
- ↑ Alström, P. & Ranft, R. (2003). "The use of sounds in avian systematics, and the importance of bird sound archives". Bulletin of the British Ornithologists' Club Supplement. 123A: 114–135.
- ↑ Alström, P. (1998). "Taxonomy of the Mirafra assamica complex" (PDF). Forktail. 13: 97–107.
- ↑ "App - Bird Song Id USA: Nature Apps - Sunbird". sunbird.tv. Retrieved 2016-06-08.
- ↑ "Help & FAQs | Merlin Bird ID app – Instant Bird Identification Help for 400 North American birds". merlin.allaboutbirds.org. Retrieved 2016-06-08.
- ↑ "Don't know birdsong? There's a (great) app for that". Telegraph.co.uk. Retrieved 2016-06-08.
- 1 2 "Archive Pioneers - Ludwig Koch and the Music of Nature". BBC Archives. BBC. 2009-04-15. Retrieved 2 September 2011.
- ↑ Collias, N. E. (1987). "The vocal repertoire of the Red Junglefowl: A spectrographic classification and the code of communication". The Condor. 89 (3): 510–524. doi:10.2307/1368641. JSTOR 1368641.
- ↑ Evans, C. S.; Macedonia, J. M.; Marler, P. (1993). "Effects of apparent size and speed on the response of chickens, Gallus gallus, to computer-generated simulations of aerial predators". Animal Behaviour. 46: 1–11. doi:10.1006/anbe.1993.1156.
- ↑ Pepperberg, I.M. (2000). The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots. Harvard University Press.
- ↑ Marcus, Gary F. (2006-04-27). "Language: Startling starlings". Nature. 440 (7088): 1117–1118. doi:10.1038/4401117a. PMID 16641976.
- ↑ Young, Jon (2008). "Bird Language: Exploring the Language of Nature with Jon Young". OWLink Media.
- ↑ Matthew Head (1997). "Birdsong and the Origins of Music". Journal of the Royal Musical Association. 122 (1): 1–23. doi:10.1093/jrma/122.1.1.
- ↑ Clark, Suzannah (2001). Music Theory and Natural Order from the Renaissance to the Early Twentieth Century. Cambridge University Press. ISBN 0-521-77191-9.
- ↑ Reich, Ronni (15 October 2010). "NJIT professor finds nothing cuckoo in serenading our feathered friends". Star Ledger. Retrieved 19 June 2011.
- ↑ Rothenberg, David (2005). Why Birds Sing. Allen Lane.
- ↑ Motion, Andrew (10 December 2005). "In full flight". The Guardian. Retrieved 24 April 2016.
- ↑ "Why Birds Sing". British Broadcasting Corporation (BBC Four). 1 November 2010. Retrieved 24 April 2016.
- ↑ Underwood, Emily (15 August 2016). "Birdsong Not Music, After All". Science. Retrieved 24 April 2016.
- ↑ Baptista, Luis Felipe; Keister, Robin A. (2005). "Why Birdsong is Sometimes Like Music". Perspectives in Biology and Medicine. 48 (3): 426–443. doi:10.1353/pbm.2005.0066.
- ↑ Tierney, Adam T.; Russo, Frank A.; Patel, Aniruddh D. (2011). "The motor origins of human and avian song structure" (PDF). PNAS. 108 (37): 15510–15515. doi:10.1073/pnas.1103882108.
- ↑ Attar, Farid al-Din (1984). Darbandi, Afkham; Davis, Dick, eds. The Conference of the Birds. Penguin Classics. ISBN 978-0-14-044434-6.
- ↑ Brooks, Cleanth; Warren, Robert Penn (1968). Stillinger, Jack, ed. The Ode to a Nightingale. Keats's Odes. Prentice-Hall. pp. 44–47.
- ↑ Sandy, Mark (2002). "To a Skylark". The Literary Encyclopedia. Retrieved 22 April 2016.
- ↑ "Crow - The Ted Hughes Society Journal". The Ted Hughes Society. 2012. Retrieved 22 April 2016.
- ↑ Hopkins, Gerard Manley (1985). Poems and Prose. Penguin Books.
External links
| Wikimedia Commons has media related to Sounds of birds. |
- The Nature Explorers Audio and video of Western North American birds.
- Bird Language: Exploring the Language of Nature with Jon Young A blog with stories and tips for learning the patterns in bird vocalizations.
- Large collection of audio bird calls collected in Arizona from Ask A Biologist.
- xeno-canto: Community online database of downloadable bird sounds from around the globe ≈250,000 recordings of ≈9400 species as of Aug 2015. See also xeno-canto.
- British Library's archive of bird sounds representing more than 8,000 species.
- Listen to Nature includes article "The Language of Birds"
- Bird language articles
- Bird songs in movies: an unnatural history Humor piece on soundtrack errors
- How do Birds Sing? The mechanics and anatomy of birdsong production
- Song Bird Science Shared resource for birdsong scientists
- Bioacoustic Research Program at the Cornell Lab of Ornithology distributes a number of different free birdsong synthesis & analysis programs.
- Macaulay Library at the Cornell Lab of Ornithology is the world's largest collection of animal sounds and associated video.
- Male shama songs and mimic of sounds
- Audio Pitch Tracer Accurate transcription of clean recordings of bird vocalizations to midi
