Benzofuran
This article is about the heterocyclic chemical compound. For its purportedly recreational derivative drug, nicknamed "Benzo Fury", see 6-APB.
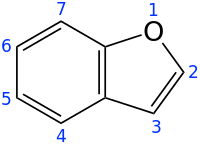 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Benzofuran[1] | |||
| Other names | |||
| Identifiers | |||
| 271-89-6 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:35260 | ||
| ChEMBL | ChEMBL363614 | ||
| ChemSpider | 8868 | ||
| DrugBank | DB04179 | ||
| ECHA InfoCard | 100.005.439 | ||
| KEGG | C14512 | ||
| PubChem | 9223 | ||
| UNII | LK6946W774 | ||
| |||
| |||
| Properties | |||
| C8H6O | |||
| Molar mass | 118.14 g·mol−1 | ||
| Melting point | −18 °C (0 °F; 255 K) | ||
| Boiling point | 173 °C (343 °F; 446 K) | ||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
500 mg/kg (mice).[2] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Production
Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol.[2]
Laboratory methods
Benzofurans can be prepared by various methods in the laboratory. Notable examples include:
- O-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration of the resulting ether.[3]

- Diels–Alder reaction of nitro vinyl furans with various dienophiles:[7]
Related compounds
- Substituted benzofurans
- Furan, an analog without the fused benzene ring.
- Indole, an analog with a nitrogen instead of the oxygen atom.
- Benzothiophene, an analog with a sulfur instead of the oxygen atom.
- Isobenzofuran, the isomer with oxygen in the adjacent position.
- Aurone
- Thunberginol F
References
- 1 2 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 218. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 Collin, G.; Höke, H. (2007). "Benzofurans". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.l03_l01.
- ↑ Burgstahler, A. W.; Worden, L. R. (1966). "Coumarone" (PDF). Org. Synth. 46: 28.; Coll. Vol., 5, p. 251
- ↑ Perkin, W. H. (1870). "XXIX. On some New Bromine Derivatives of Coumarin". Journal of the Chemical Society. 23: 368–371. doi:10.1039/JS8702300368.
- ↑ Perkin, W. H. (1871). "IV. On some New Derivatives of Coumarin". Journal of the Chemical Society. 24: 37–55. doi:10.1039/JS8712400037.
- ↑ Bowden, K.; Battah, S. (1998). "Reactions of Carbonyl Compounds in Basic Solutions. Part 32. The Perkin Rearrangement". Journal of the Chemical Society, Perkin Transactions 2. 1998 (7): 1603–1606. doi:10.1039/a801538d.
- ↑ Kusurkar, R. S.; Bhosale, D. K. (1990). "Novel Synthesis of Benzosubstituted Benzofurans Via Diels-Alder Reaction". Synthetic Communications. 20 (1): 101–109. doi:10.1080/00397919008054620. Retrieved 11 February 2014.
- ↑ Fürstner, Alois & Davies, Paul (2005). "Heterocycles by PtCl2-Catalyzed Intramolecular Carboalkoxylation or Carboamination of Alkynes". Journal of the American Chemical Society. 127 (43): 15024–15025. doi:10.1021/ja055659p. Retrieved 11 February 2014.
This article is issued from Wikipedia - version of the 9/19/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.