Benign prostatic hyperplasia
| Benign prostatic hyperplasia | |
|---|---|
| adenofibromyomatous hyperplasia, benign prostatic hypertrophy | |
|
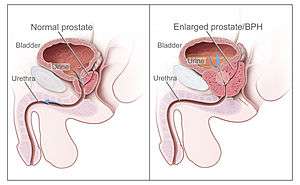
Diagram illustrating normal prostate (left) and benign prostatic hyperplasia (right). | |
| Classification and external resources | |
| Specialty | Urology |
| ICD-10 | N40 |
| ICD-9-CM | 600 |
| OMIM | 600082 |
| DiseasesDB | 10797 |
| MedlinePlus | 000381 |
| eMedicine | med/1919 |
| Patient UK | Benign prostatic hyperplasia |
| MeSH | D011470 |
Benign prostatic hyperplasia (BPH), also called benign enlargement of the prostate (BEP or BPE), is a noncancerous increase in size of the prostate. BPH involves hyperplasia of prostatic stromal and epithelial cells, resulting in the formation of large, fairly discrete nodules in the transition zone of the prostate.[1] When sufficiently large, the nodules impinge on the urethra and increase resistance to flow of urine from the bladder. This is commonly referred to as "obstruction," although the urethral lumen is no less patent, only compressed. Resistance to urine flow requires the bladder to work harder during voiding, possibly leading to progressive hypertrophy, instability, or weakness (atony) of the bladder muscle. BPH involves hyperplasia (an increase in the number of cells) rather than hypertrophy (a growth in the size of individual cells), but the two terms are often used interchangeably, even among urologists.[2] Although prostate specific antigen levels may be elevated in these patients because of increased organ volume and inflammation due to urinary tract infections, BPH does not lead to cancer or increase the risk of cancer.[3]
Adenomatous prostatic growth is believed to begin at approximately age 30. An estimated 50% of men have histologic evidence of BPH by age 50 and 75% by age 80; in 40–50% of these men, BPH becomes clinically significant.[4] BPH was one of the ten most prominent and costly diseases in men older than 50 years of age in a study in the United States.[5]
Signs and symptoms
.png)
BPH is the most common cause of lower urinary tract symptoms (LUTS), which are divided into storage, voiding, and symptoms which occur after urination.[6] Storage symptoms include the need to urinate frequently, waking at night to urinate, urgency (compelling need to void that cannot be deferred), involuntary urination, including involuntary urination at night, or urge incontinence (urine leak following a strong sudden need to urinate).[7] Voiding symptoms include urinary hesitancy (a delay between trying to urinate and the flow actually beginning), intermittency (not continuous),[8] involuntary interruption of voiding, weak urinary stream, straining to void, a sensation of incomplete emptying, and terminal dribbling (uncontrollable leaking after the end of urination, also called post-micturition dribbling).[9][10][11] These symptoms may be accompanied by bladder pain or pain while urinating, called dysuria.[12]
Bladder outlet obstruction (BOO) can be caused by BPH.[13] Symptoms are abdominal pain, a continuous feeling of a full bladder, frequent urination, acute urinary retention (inability to urinate), pain during urination (dysuria), problems starting urination (urinary hesitancy), slow urine flow, starting and stopping (urinary intermittence), and nocturia.
BPH can be a progressive disease, especially if left untreated. Incomplete voiding results in residual urine or urinary stasis, which can lead to an increased risk of urinary tract infection.
Symptoms can vary throughout the day with mild symptoms after standing or walking and more pronounced symptoms after lying down.
Causes
Most experts consider androgens (testosterone and related hormones) to play a permissive role in the development of BPH. This means that androgens have to be present for BPH to occur, but do not necessarily directly cause the condition. This is supported by evidence suggesting that castrated boys do not develop BPH when they age. In an unusual study of 26 eunuchs from the palace of the Qing dynasty still living in Beijing in 1960, the prostate was impalpable (could not be felt) in 81%.[14] The average time since castration was 54 years (range, 41–65 years). On the other hand, some studies suggest that administering exogenous testosterone is not associated with a significant increase in the risk of BPH symptoms, so the role of testosterone in prostate cancer and BPH is still unclear. Further randomized controlled trials with more participants are needed to quantify any risk of giving exogenous testosterone.[15]
Dihydrotestosterone (DHT), a metabolite of testosterone, is a critical mediator of prostatic growth. DHT is synthesized in the prostate from circulating testosterone by the action of the enzyme 5α-reductase, type 2. This enzyme is localized principally in the stromal cells; hence, those cells are the main site for the synthesis of DHT. DHT can act in an autocrine fashion on the stromal cells or in paracrine fashion by diffusing into nearby epithelial cells. In both of these cell types, DHT binds to nuclear androgen receptors and signals the transcription of growth factors that are mitogenic to the epithelial and stromal cells. DHT is ten times more potent than testosterone because it dissociates from the androgen receptor more slowly. The importance of DHT in causing nodular hyperplasia is supported by clinical observations in which an inhibitor of 5α-reductase such as finasteride is given to men with this condition. Therapy with a 5α-reductase inhibitor markedly reduces the DHT content of the prostate and, in turn, reduces prostate volume and BPH symptoms.[16][17]
Testosterone promotes prostate cell proliferation,[18] but relatively low levels of serum testosterone are found in patients with BPH.[19][20] One small study has shown that medical castration lowers the serum and prostate hormone levels unevenly, having less effect on testosterone and dihydrotestosterone levels in the prostate.[21]
While there is some evidence that estrogen may play a role in the etiology of BPH, this effect appears to be mediated mainly through local conversion of androgens to estrogen in the prostate tissue rather than a direct effect of estrogen itself.[22] In canine in vivo studies castration, which significantly reduced androgen levels but left estrogen levels unchanged, caused significant atrophy of the prostate.[23] Studies looking for a correlation between prostatic hyperplasia and serum estrogen levels in humans have generally shown none.[20][24]
In 2008, Gat et al. published evidence that BPH is caused by failure in the spermatic venous drainage system resulting in increased hydrostatic pressure and local testosterone levels elevated more than 100 fold above serum levels.[25] If confirmed, this mechanism explains why serum androgen levels do not seem to correlate with BPH and why giving exogenous testosterone would not make much difference. This also has implications for treatment (see Minimally invasive therapies below).
Studies indicate that dietary patterns may affect development of BPH, but further research is needed to clarify any important relationship.[26] Studies from China suggest that greater protein intake may be a factor in development of BPH. Men older than 60 in rural areas had very low rates of clinical BPH, while men living in cities and consuming more animal protein had a higher incidence.[27][28] On the other hand, a study in Japanese-American men found a strong association with alcohol intake, but a weak association with beef intake.[29] In a large prospective cohort study in the US (the Health Professionals Follow-up Study), investigators reported modest associations between BPH (men with strong symptoms of BPH or surgically confirmed BPH) and total energy and protein, but not fat intake.[30] There is also epidemiological evidence linking BPH with metabolic syndrome (concurrent obesity, impaired glucose metabolism and diabetes, hypertriglyceridemia, low-density cholesterol and hypertension).[31]
Benign prostatic hyperplasia is an age-related disease. Misrepair-accumulation aging theory[32][33] suggests that development of benign prostatic hyperplasia is a consequence of fibrosis and weakening of the muscular tissue in the prostate.[34] The muscular tissue is important in the functionality of the prostate, and provides the force for excreting the fluid produced by prostatic glands. However, repeated contractions and dilations of myofibers will unavoidably cause injuries and broken myofibers. Myofibers have low potential of regeneration; therefore collagen fibers need to be used to replace the broken myofibers. Such misrepairs make the muscular tissue weak in functioning, and the fluid secreted in glands cannot be excreted completely. Then, the accumulation of fluid in glands increases the resistance of muscular tissue during the movements of contractions and dilations, and more and more myofibers will be broken and replaced by collagen fibers. Progressive fibrosis of muscular tissue and accumulation of fluid are important causes for the expanding of the prostate in benign prostatic hyperplasia.
Pathophysiology
An increase in age leads to an increase of the enzymes aromatase and 5-alpha reductase. Aromatase and 5-alpha reductase are responsible for converting androgen hormones into estrogen and dihydrotestosterone, respectively. This metabolism of androgen hormones leads to a decrease in testosterone but raised levels of DHT and estrogen. Estrogen has a key role in the growth of cells in the prostate and DHT is an anabolic hormone many times more potent than testosterone that when combined, cause a synergy to induce BPH.
Both the glandular epithelial cells and the stromal cells (including muscular fibers) undergo hyperplasia in BPH.[35]:694 Most sources agree that of the two tissues, stromal hyperplasia predominates, but the exact ratio of the two is unclear.[35]:694
Anatomically the median lobe is usually enlarged in BPH. The anterior lobe has little in the way of glandular tissue and is seldom enlarged. (Carcinoma of the prostate typically occurs in the posterior lobe – hence the ability to discern an irregular outline per rectal examination). The earliest microscopic signs of BPH usually begin between the age of 30 and 50 years old in the PUG, which are posterior to the proximal urethra.[35]:694 In BPH, the majority of growth occurs in the TZ.[35]:694 In addition to these two classic areas, the peripheral zone (PZ) of the prostate is also involved to a lesser extent.[35]:695 Prostatic cancer typically occurs in the PZ. However, BPH nodules, usually from the TZ are often biopsied anyway to rule out cancer in the TZ.[35]:695 However, cancers of the prostate most frequently occur in the PZ rather than the TZ, thus, chippings taken from the PZ are of limited use.
Diagnosis
BPH is diagnosed using the American Urological Association Symptom Index (AUA-SI), the internationally validated counterpart, the International Prostate Symptom Score (I-PSS),[36] and more recently the UWIN score (urgency, weak stream, incomplete emptying, and nocturia).[37] An IPSS score <7 is "mildly symptomatic" and does not usually require pharmacotherapy.
Screening and diagnostic procedures for BPH are similar to those used for prostate cancer.[38]
The clinical diagnosis is based on a history of LUTS, a digital rectal exam, and exclusion of other causes. The degree of LUTS does not necessarily correspond to the size of the prostate. Rectal examination (palpation of the prostate through the rectum) may reveal a markedly enlarged prostate, usually affecting the middle lobe. Blood tests are often performed to rule out prostatic malignancy. Elevated prostate specific antigen (PSA) levels need further evaluation, such as reinterpretation of PSA results, in terms of PSA density and PSA free percentage, rectal examination and transrectal ultrasonography. These combined measures can provide early detection. Ultrasound examination of the testicles, prostate, and kidneys is often performed, again to rule out malignancy and hydronephrosis.
The differential diagnosis includes other diseases of the bladder, urethra, and prostate such as bladder cancer, urinary tract infection, urethral stricture, urethral calculi (stones), chronic prostatitis and prostate cancer.
-

Urinary bladder (black butterfly-like shape) and hyperplastic prostate (BPH) visualized by sonography
-

Micrograph showing nodular hyperplasia (left off center) of the prostate from a transurethral resection of the prostate (TURP). H&E stain.
-
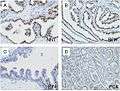
Microscopic examination of different types of prostate tissues (stained with immunohistochemical techniques): A. Normal (non-neoplastic) prostatic tissue (NNT). B. Benign prostatic hyperplasia. C. High-grade prostatic intraepithelial neoplasia (PIN). D. Prostatic adenocarcinoma (PCA).
Management
Lifestyle
Lifestyle alterations to address the symptoms of BPH include physical activity,[39] decreasing fluid intake before bedtime, moderating the consumption of alcohol and caffeine-containing products, and following a timed voiding schedule. Patients can also attempt to avoid products and medications with anticholinergic properties that may exacerbate urinary retention symptoms of BPH, including antihistamines, decongestants, opiates, and tricyclic antidepressants; however, changes in medications should be done with input from a medical professional.[40]
Voiding position
Voiding position when urinating may influence urodynamic parameters (urinary flow rate, voiding time, and post-void residual volume).[41] A meta-analysis found no differences between the standing and sitting positions for healthy males, but that, for elderly males with lower urinary tract symptoms (LUTS), voiding in the sitting position:[42]
- the post void residual volume was significantly decreased
- the maximum urinary flow was increased, comparable with pharmacological intervention
- the voiding time was decreased
This urodynamic profile is associated with a lower risk of urologic complications, such as cystitis and bladder stones.
Medications
The two main medications for management of BPH are alpha blockers and 5α-reductase inhibitors.[43]
Selective α1-blockers are the most common choice for initial therapy.[44][45][46] They include doxazosin,[47] terazosin, alfuzosin,[48][49] tamsulosin, and silodosin. They have a small to moderate benefit.[50] All five are equally effective but have slightly different side effect profiles.[51] Alfuzosin, tamsulosin and silodosin are selective α1 receptor antagonist that have preferential selectivity for the α1A receptor in the prostate versus the α1Breceptor in the blood vessels. Less-selective α1 receptor antagonists such as terazosin and doxazosin may lower blood pressure. The older less selective α1-adrenergic blocker prazosin is not a first line choice for either hypertension or prostatic hyperplasia; it is a choice for patients who present with both problems at the same time. The older broadly non-selective alpha blocker medications such as phenoxybenzamine are not recommended for control of BPH.[52]
Alpha blockers relax smooth muscle in the prostate and the bladder neck, thus decreasing the blockage of urine flow. Common side effects of alpha blockers include orthostatic hypotension, (a head rush or dizzy spell when standing up or stretching), ejaculation changes, headaches, nasal congestion, and weakness. Non-selective alpha blockers such as terazosin and doxazosin may also require titration (gradually adjusting the dose of a medication) as they can lower blood pressure and cause syncope (fainting) if the response is too high. Side effects can also include erectile dysfunction.[53]
The 5-alpha-reductase inhibitors finasteride[54] and dutasteride[55] may also be used in men with BPH. These medications inhibit the 5a-reductase enzyme, which in turn inhibits production of DHT, a hormone responsible for enlarging the prostate. Effects may take longer to appear than alpha blockers, but they persist for many years.[56] When used together with alpha blockers, no benefit was reported in short-term trials, but in a longer term study (3–4 years) there was a greater reduction in BPH progression to acute urinary retention and surgery than with either agent alone, especially in patients were more severe symptoms and larger prostates.[57][58][59] Other trials have confirmed reductions in symptoms, within 6 months in one trial, an effect that was maintained after withdrawal of the alpha-blocker.[58][60] Side effects include decreased libido and ejaculatory or erectile dysfunction.[54][61] The 5-alpha-reductase inhibitors are contraindicated in pregnant women because of the interference with testosterone metabolism, and as a precaution, pregnant women should not handle crushed or broken tablets.[62]
Antimuscarinics such as tolterodine may also be used, especially in combination with alpha blockers.[63] They act by decreasing acetylcholine effects on the smooth muscle of the bladder, thus helping control symptoms of an overactive bladder.[64]
Sildenafil citrate shows some symptomatic relief, suggesting a possible common cause with erectile dysfunction.[65] Tadalafil was considered then rejected by NICE in the UK for the treatment of symptoms associated with BPH.[66] In 2011, the U.S. Food and Drug Administration approved tadalafil to treat the signs and symptoms of benign prostatic hyperplasia, and for the treatment of BPH and erectile dysfunction (ED), when the conditions occur simultaneously.[67]
Self-catheterization
Intermittent urinary catheterization is used to relieve the bladder in people with urinary retention. Self-catheterization is an option in BPH when the bladder is difficult or impossible to completely empty.[68] Urinary tract infection is the most common complication of intermittent catheterization.[69] Several techniques and types of catheter are available, including sterile (single-use) and clean (multiple use) catheters, but none is superior to others in reducing the incidence of urinary tract infection based on current information.[70]
Surgery

If medical treatment is not effective a person may try office-based therapies or transurethral resection of prostate (TURP), surgery may need to be performed. Surgical techniques used include
- Open prostatectomy: not usually performed nowadays, even if results are very good.
- Trans-urethral resection of the prostate (TURP): the gold standard.
- Transurethral incision of the prostate (TUIP): rarely performed; the technique is similar to TURP but less definitive.
- Photoselective (laser) vaporization of the prostate (PVP): common treatment.
- Transurethral microwave therapy (TUMT): similar to laser ablation, but less effective and much less used.
- Transurethral needle ablation (TUNA): not very effective.
- Holmium laser enucleation of the prostate (HoLEP): more and more used, it will probably replace TURP in the future.
Alternative medicine
Herbal remedies are commonly used,[71] and several are approved in European countries and available in the USA. Saw palmetto extract from Serenoa repens is one of the most commonly used and studied, having shown some promise in early studies.[72] Later trials of higher methodological quality have shown it to be no better than placebo in both symptom relief and decreasing prostate size.[73][74][75] Other herbal medicines include beta-sitosterol[76] from Hypoxis rooperi (African star grass) and pygeum (extracted from the bark of Prunus africana),[77] while there is less substantial support for the efficacy of pumpkin seed (Cucurbita pepo) and stinging nettle (Urtica dioica) root.[78] In a 2016 review, all most popular herbal remedies have been shown to be no more efficacious than placebo.[79] A systematic review of Chinese herbal medicines found that the quality of studies was insufficient to indicate any superiority over Western medicines.[80]
Epidemiology
Globally, benign prostatic hyperplasia affects about 210 million males as of 2010 (6% of the population).[82] The prostate gets larger in most men as they get older. For a symptom-free man of 46 years, the risk of developing BPH over the next 30 years is 45%. Incidence rates increase from 3 cases per 1000 man-years at age 45–49 years, to 38 cases per 1000 man-years by the age of 75–79 years. While the prevalence rate is 2.7% for men aged 45–49, it increases to 24% by the age of 80 years.[83]
Research
Prostatic arterial embolization is being studied as a possible treatment.[84]
References
- ↑ Cunningham GR, Kadmon D. "Epidemiology and pathogenesis of benign prostatic hyperplasia." UpToDate; updated Sep. 10, 2013
- ↑ Bostwick, D. G. (2002). "The Pathology of Benign Prostatic Hyperplasia". In Kirby, Roger S.; McConnell, John D.; Fitzpatrick, John M.; Roehrborn, Claus G.; Boyle, Peter. Textbook of Benign Prostatic Hyperplasia. London: Isis Medical Media. ISBN 978-1-901865-55-4.
- ↑ "Is there a link between BPH and prostate cancer?". Practitioner. 256: 13–6, 2. Apr 2012. PMID 22792684.
- ↑ Guess, HA; Arrighi, HM; Metter, EJ; Fozard, JL (1990). "Cumulative prevalence of prostatism matches the autopsy prevalence of benign prostatic hyperplasia.". The Prostate. 17 (3): 241–6. doi:10.1002/pros.2990170308. PMID 1700403.
- ↑ Fenter, TC (2006). "The cost of treating the 10 most prevalent diseases in men 50 years of age or older.". Am J Manag Care. 12 (4 Suppl): S90-8. PMID 16551207.
- ↑ "Lower urinary tract symptoms in men: management". NICE (National Institute for Health and Care Excellence).
- ↑ "Urge incontinence". MedlinePlus. US National Library of Medicine. Retrieved 26 October 2015.
- ↑ White, JM; O'Brien, DP; Walker, HK; Hall, WD; Hurst, JW (1990). "Incontinence and Stream Abnormalities". PMID 21250138.
- ↑ Robinson, J (undefined NaN). "Post-micturition dribble in men: causes and treatment.". Nursing standard (Royal College of Nursing (Great Britain) : 1987). 22 (30): 43–6. doi:10.7748/ns2008.04.22.30.43.c6440. PMID 18459613. Check date values in:
|date=(help) - ↑ Sarma, AV; Wei, JT (19 July 2012). "Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms.". The New England Journal of Medicine. 367 (3): 248–57. doi:10.1056/nejmcp1106637. PMID 22808960.
- ↑ "Urination - difficulty with flow". MedlinePlus. US National Library of Medicine. Retrieved 26 October 2015.
- ↑ "Urination - painful". MedlinePlus. US National Library of Medicine. Retrieved 26 October 2015.
- ↑ "Bladder outlet obstruction". MedlinePlus. US National Library of Medicine. Retrieved 26 October 2015.
- ↑ Wu, CP; Gu, FL (1991). "The prostate in eunuchs". Prog Clin Biol Res. 370: 249–55. PMID 1924456.
- ↑ "Testosterone and Aging: Clinical Research Directions.". NCBI Bookshelf. Retrieved 2 February 2015.
- ↑ "Proscar (finisteride) Prescribing Information" (PDF). FDA - Drug Documents. Merck and Company. Retrieved 2 March 2015.
- ↑ Bartsch, G; Rittmaster, RS; Klocker, H (2002). "Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia". World J Urol. 19 (6): 413–25. doi:10.1007/s00345-002-0248-5. PMID 12022710.
- ↑ Feldman, Brian J.; Feldman, David (2001). "The development of androgen-independent prostate cancer". Nature Reviews Cancer. 1 (1): 34–45. doi:10.1038/35094009. PMID 11900250.
- ↑ Lagiou, Pagona; Mantzoros, Christos S.; Tzonou, Anastasia; Signorello, Lisa B.; Lipworth, Loren; Trichopoubs, Dimitrios (1997). "Serum Steroids in Relation to Benign Prostatic Hyperplasia". Oncology. 54 (6): 497–501. doi:10.1159/000227609. PMID 9394847.
- 1 2 Roberts, Rosebud O.; Jacobson, Debra J.; Rhodes, Thomas; Klee, George G.; Leiber, Michael M.; Jacobsen, Steven J. (2004). "Serum sex hormones and measures of benign prostatic hyperplasia". The Prostate. 61 (2): 124–31. doi:10.1002/pros.20080. PMID 15305335.
- ↑ Page, S. T.; Lin, D. W.; Mostaghel, E. A.; Hess, D. L.; True, L. D.; Amory, J. K.; Nelson, P. S.; Matsumoto, A. M.; Bremner, W. J. (2006). "Persistent Intraprostatic Androgen Concentrations after Medical Castration in Healthy Men". Journal of Clinical Endocrinology & Metabolism. 91 (10): 3850–6. doi:10.1210/jc.2006-0968. PMID 16882745.
- ↑ Ho, C. K M; Nanda, J.; Chapman, K. E; Habib, F. K (2008). "Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen". Journal of Endocrinology. 197 (3): 483–91. doi:10.1677/JOE-07-0470. PMID 18492814.
- ↑ Niu, YJ; Ma, TX; Zhang, J; Xu, Y; Han, RF; Sun, G (2003). "Androgen and prostatic stroma". Asian Journal of Andrology. 5 (1): 19–26. PMID 12646998.
- ↑ Ansari, Mohammad Abduljalil; Begum, Dilruba; Islam, Fakhrul (2008). "Serum sex steroids, gonadotrophins and sex hormone-binding globulin in prostatic hyperplasia". Annals of Saudi Medicine. 28 (3): 174–8. doi:10.4103/0256-4947.51727. PMID 18500180.
- ↑ Gat, Y; Gornish, M; Heiblum, M; Joshua, S (2008). "Reversal of benign prostate hyperplasia by selective occlusion of impaired venous drainage in the male reproductive system: novel mechanism, new treatment". Andrologia. 40 (5): 273–281. doi:10.1111/j.1439-0272.2008.00883.x. PMID 18811916.
- ↑ Heber, D (2002). "Prostate enlargement: the canary in the coal mine?". Am J Clin Nutr. 75 (4): 605–6. PMID 11916745.
- ↑ Zhang, SX; Yu, B; Guo, SL; Wang, YW; Yin, CK (February 2003). "[Comparison of incidence of BPH and related factors between urban and rural inhabitants in district of Wannan].". Zhonghua nan ke xue = National journal of andrology. 9 (1): 45–7. PMID 12680332.
- ↑ Gu, F (March 1997). "Changes in the prevalence of benign prostatic hyperplasia in China.". Chinese medical journal. 110 (3): 163–6. PMID 9594331.
- ↑ Chyou, PH (1993). "A prospective study of alcohol, diet, and other lifestyle factors in relation to obstructive uropathy". Prostate. 22 (3): 253–64. doi:10.1002/pros.2990220308. PMID 7683816.
- ↑ Suzuki, S (2002). "Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia". Am J Clin Nutr. 75 (4): 689–97. PMID 11916755.
- ↑ Gacci, M (2015). "Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis". BJU International. 115 (1): 24–31. doi:10.1111/bju.12728. PMID 24602293. Retrieved 1 March 2015.
- ↑ Wang, Jicun; Michelitsch, Thomas; Wunderlin, Arne; Mahadeva, Ravi (2009). "Aging as a consequence of Misrepair –a novel theory of aging". 0904 (0575). arXiv:0904.0575
 . Bibcode:2009arXiv0904.0575W.
. Bibcode:2009arXiv0904.0575W. - ↑ Wang-Michelitsch, Jicun; Michelitsch, Thomas (2015). "Aging as a process of accumulation of Misrepairs". 1503 (07163). arXiv:1503.07163
 . Bibcode:2015arXiv150307163W.
. Bibcode:2015arXiv150307163W. - ↑ Wang-Michelitsch, Jicun; Michelitsch, Thomas (2015). "Tissue fibrosis: a principal evidence for the central role of Misrepairs in aging". 1505 (01376). arXiv:1503.01376
 . Bibcode:2015arXiv150301376C.
. Bibcode:2015arXiv150301376C. - 1 2 3 4 5 6 Wasserman, Neil F. (1 September 2006). "Benign Prostatic Hyperplasia: A Review and Ultrasound Classification". Radiologic Clinics of North America. 44 (5): 689–710. doi:10.1016/j.rcl.2006.07.005. PMID 17030221.
- ↑ Parsons, JK (December 2010). "Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors.". Current bladder dysfunction reports. 5 (4): 212–218. doi:10.1007/s11884-010-0067-2. PMID 21475707.
- ↑ Eid, K; Krughoff, K; Stoimenova, D; Smith, D; Phillips, J; O'Donnell, C; Barqawi, A (January 2014). "Validation of the Urgency, Weak stream, Incomplete emptying, and Nocturia (UWIN) score compared with the American Urological Association Symptoms Score in assessing lower urinary tract symptoms in the clinical setting.". Urology. 83 (1): 181–5. doi:10.1016/j.urology.2013.08.039. PMID 24139351.
- ↑ Janis, Craitlyn (19 May 2008). "A Brief Overview of Benign Prostatic Hyperplasia (BPH)". Associated Content.
- ↑ Silva V, Grande AJ, Stanton KR, Peccin MS. Physical activity for lower urinary tract symptoms secondary to benign prostatic obstruction (Protocol). Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No.: CD012044. DOI: 10.1002/14651858.CD012044.
- ↑ "Benign prostatic hyperplasia". University of Maryland Medical Center.
- ↑ Y. de Jong; R.M. ten Brinck; J.H.F.M. Pinckaers; A.A.B. Lycklama à Nijeholt. "Influence of voiding posture on urodynamic parameters in men: a literature review" (PDF). Nederlands Tijdschrift voor urologie). Retrieved 2014-07-02.
- ↑ de Jong, Y; Pinckaers, JH; Ten Brinck, RM; Lycklama À Nijeholt, AA; Dekkers, OM (2014). "Urinating Standing versus Sitting: Position Is of Influence in Men with Prostate Enlargement. A Systematic Review and Meta-Analysis.". PLOS ONE. 9 (7): e101320. doi:10.1371/journal.pone.0101320. PMC 4106761
 . PMID 25051345.
. PMID 25051345. - ↑ Silva, J; Silva, CM; Cruz, F (January 2014). "Current medical treatment of lower urinary tract symptoms/BPH: do we have a standard?". Current opinion in urology. 24 (1): 21–8. doi:10.1097/mou.0000000000000007. PMID 24231531.
- ↑ Roehrborn, Claus G.; Nuckolls, James G.; Wei, John T.; Steers, William; BPH Registry and Patient Survey Steering Committee (2007). "The Benign Prostatic Hyperplasia Registry and Patient Survey: study design, methods and patient baseline characteristics". BJU International. 100 (4): 813–9. doi:10.1111/j.1464-410X.2007.07061.x. PMID 17822462.
- ↑ Black, L; Naslund, MJ; Gilbert Jr, TD; Davis, EA; Ollendorf, DA (2006). "An examination of treatment patterns and costs of care among patients with benign prostatic hyperplasia". The American journal of managed care. 12 (4 Suppl): S99–S110. PMID 16551208.
- ↑ Hutchison, A; Farmer, R; Verhamme, K; Berges, R; Navarrete, R (2007). "The Efficacy of Drugs for the Treatment of LUTS/BPH, A Study in 6 European Countries". European Urology. 51 (1): 207–15 discussion 215–6. doi:10.1016/j.eururo.2006.06.012. PMID 16846678.
- ↑ MacDonald, Roderick; Wilt, Timothy J.; Howe, R. William (2004). "Doxazosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects". BJU International. 94 (9): 1263–70. doi:10.1111/j.1464-410X.2004.05154.x. PMID 15610102.
- ↑ MacDonald, Roderick; Wilt, Timothy J. (2005). "Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: A systematic review of efficacy and adverse effects". Urology. 66 (4): 780–8. doi:10.1016/j.urology.2005.05.001. PMID 16230138.
- ↑ Roehrborn, Claus G (2001). "Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial". Urology. 58 (6): 953–9. doi:10.1016/S0090-4295(01)01448-0. PMID 11744466.
- ↑ Wilt, TJ; Mac Donald, R; Rutks, I (2003). "Tamsulosin for benign prostatic hyperplasia.". The Cochrane database of systematic reviews (1): CD002081. doi:10.1002/14651858.CD002081. PMID 12535426.
- ↑ Djavan, Bob; Marberger, Michael (1999). "A Meta-Analysis on the Efficacy and Tolerability of α1-Adrenoceptor Antagonists in Patients with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Obstruction". European Urology. 36 (1): 1–13. doi:10.1159/000019919. PMID 10364649.
- ↑ Aua Practice Guidelines, Committee (2003). "AUA Guideline on Management of Benign Prostatic Hyperplasia (2003). Chapter 1: Diagnosis and Treatment Recommendations". The Journal of Urology. 170 (2 Pt 1): 530–47. doi:10.1097/01.ju.0000078083.38675.79. PMID 12853821.
- ↑ Santillo, VM; Lowe FC (2006). "Treatment of benign prostatic hyperplasia in patients with cardiovascular disease". Drugs and Aging. 10 (23): 795–805. doi:10.2165/00002512-200623100-00003. PMID 17067183.
- 1 2 Gormley, Glenn J.; Stoner, Elizabeth; Bruskewitz, Reginald C.; Imperato-Mcginley, Julianne; Walsh, Patrick C.; McConnell, John D.; Andriole, Gerald L.; Geller, Jack; et al. (1992). "The Effect of Finasteride in Men with Benign Prostatic Hyperplasia". New England Journal of Medicine. 327 (17): 1185–91. doi:10.1056/NEJM199210223271701. PMID 1383816.
- ↑ Roehrborn, C; Boyle, P; Nickel, JC; Hoefner, K; Andriole, G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators (2002). "Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia". Urology. 60 (3): 434–41. doi:10.1016/S0090-4295(02)01905-2. PMID 12350480.
- ↑ Roehrborn, C; Bruskewitz, R; Nickel, J; McConnell, J; Saltzman, B; Gittelman, M; Malek, G; Gottesman, J; et al. (2004). "Sustained Decrease in Incidence of Acute Urinary Retention and Surgery With Finasteride for 6 Years in Men With Benign Prostatic Hyperplasia". The Journal of Urology. 171 (3): 1194–8. doi:10.1097/01.ju.0000112918.74410.94. PMID 14767299.
- ↑ Roehrborn, CG; Barkin, J; Tubaro, A; Emberton, M; Wilson, TH; Brotherton, BJ; Castro, R (April 2014). "Influence of baseline variables on changes in International Prostate Symptom Score after combined therapy with dutasteride plus tamsulosin or either monotherapy in patients with benign prostatic hyperplasia and lower urinary tract symptoms: 4-year results of the CombAT study.". BJU international. 113 (4): 623–35. doi:10.1111/bju.12500. PMID 24127818.
- 1 2 Greco, KA; McVary, KT (December 2008). "The role of combination medical therapy in benign prostatic hyperplasia.". International Journal of Impotence Research. 20 Suppl 3: S33–43. doi:10.1038/ijir.2008.51. PMID 19002123.
- ↑ Kaplan, S; McConnell, J; Roehrborn, C; Meehan, A; Lee, M; Noble, W; Kusek, J; Nybergjr, L; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group (2006). "Combination Therapy With Doxazosin and Finasteride for Benign Prostatic Hyperplasia in Patients With Lower Urinary Tract Symptoms and a Baseline Total Prostate Volume of 25 Ml or Greater". The Journal of Urology. 175 (1): 217–20; discussion 220–1. doi:10.1016/S0022-5347(05)00041-8. PMID 16406915.
- ↑ Barkin, J; Guimarães, M; Jacobi, G; Pushkar, D; Taylor, S; van Vierssen Trip, OB (October 2003). "Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride.". European Urology. 44 (4): 461–6. doi:10.1016/s0302-2838(03)00367-1. PMID 14499682.
- ↑ Gacci, M; Ficarra, V; Sebastianelli, A; Corona, G; Serni, S; Shariat, SF; Maggi, M; Zattoni, F; Carini, M; Novara, G (June 2014). "Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: a systematic review and meta-analysis.". The journal of sexual medicine. 11 (6): 1554–66. doi:10.1111/jsm.12525. PMID 24708055.
- ↑ Deters, Levi. "Benign Prostatic Hypertrophy Treatment & Management". Medscape. Retrieved 14 November 2015.
- ↑ Kaplan, S. A.; Roehrborn, C. G.; Rovner, E. S.; Carlsson, M.; Bavendam, T.; Guan, Z. (2006). "Tolterodine and Tamsulosin for Treatment of Men With Lower Urinary Tract Symptoms and Overactive Bladder: A Randomized Controlled Trial". JAMA: The Journal of the American Medical Association. 296 (19): 2319–28. doi:10.1001/jama.296.19.2319. PMID 17105794.
- ↑ Abrams, P; Andersson, KE (November 2007). "Muscarinic receptor antagonists for overactive bladder.". BJU international. 100 (5): 987–1006. doi:10.1111/j.1464-410x.2007.07205.x. PMID 17922784.
- ↑ McVary, Kevin T.; Monnig, William; Camps Jr., Joseph L.; Young, Jay M.; Tseng, Li-Jung; Van Den Ende, Gene (2007). "Sildenafil Citrate Improves Erectile Function and Urinary Symptoms in Men With Erectile Dysfunction and Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Randomized, Double-Blind Trial". The Journal of Urology. 177 (3): 1071–7. doi:10.1016/j.juro.2006.10.055. PMID 17296414.
- ↑ "Hyperplasia (benign prostatic) - tadalafil (terminated appraisal) (TA273)". National Institute for Health and Clinical Excellence (NICE). Retrieved 27 January 2013.
- ↑ "FDA approves Cialis to treat benign prostatic hyperplasia". U.S. Food and Drug Administration (FDA). Retrieved 7 May 2013.
- ↑ "Prostate enlargement (benign prostatic hyperplasia)". Harvard Health Content. Harvard Health Publications. Retrieved 2 February 2015.
- ↑ Wyndaele, JJ (2002). "Complications of intermittent catheterization: their prevention and treatment". Spinal Cord. 40 (10): 536–41. doi:10.1038/sj.sc.3101348. PMID 12235537.
- ↑ Prieto, J; Murphy, CL; Moore, KN; Fader, M (Sep 10, 2014). "Intermittent catheterisation for long-term bladder management". Cochrane Database Syst Rev. 9: CD006008. doi:10.1002/14651858.CD006008.pub3. PMID 25208303.
- ↑ Lieber, M M (1998). "Pharmacologic therapy for prostatism". Mayo Clinic Proceedings. 73 (6): 590–6. doi:10.4065/73.6.590. PMID 9621869.
- ↑ Boyle, P; Robertson, C; Lowe, F; Roehrborn, C (2000). "Meta-analysis of clinical trials of Permixon in the treatment of symptomatic benign prostatic hyperplasia". Urology. 55 (4): 533–9. doi:10.1016/S0090-4295(99)00593-2. PMID 10736497.
- ↑ Bent, Stephen; Kane, Christopher; Shinohara, Katsuto; Neuhaus, John; Hudes, Esther S.; Goldberg, Harley; Avins, Andrew L. (2006). "Saw Palmetto for Benign Prostatic Hyperplasia". New England Journal of Medicine. 354 (6): 557–66. doi:10.1056/NEJMoa053085. PMID 16467543.
- ↑ Dedhia, R; McVary, K (2008). "Phytotherapy for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia". The Journal of Urology. 179 (6): 2119–25. doi:10.1016/j.juro.2008.01.094. PMID 18423748.
- ↑ Tacklind, J; MacDonald, R; Rutks, I; Wilt, TJ (2009). Tacklind, James, ed. "Serenoa repens for benign prostatic hyperplasia". Cochrane Database of Systematic Reviews (2): CD001423. doi:10.1002/14651858.CD001423.pub2. PMC 3090655
 . PMID 19370565.
. PMID 19370565. - ↑ Wilt, Timothy; Ishani, Areef; MacDonald, Roderick; Stark, Gerold; Mulrow, Cynthia D; Lau, Joseph; Wilt, Timothy (1999). Wilt, Timothy J, ed. "Beta-sitosterols for benign prostatic hyperplasia". Cochrane Database of Systematic Reviews (2): CD001043. doi:10.1002/14651858.CD001043. PMID 10796740.
- ↑ Wilt, Timothy; Ishani, Areef; Wilt, Timothy; Rutks, I; Stark, G (1998). Wilt, Timothy J, ed. "Pygeum africanum for benign prostatic hyperplasia". Cochrane Database of Systematic Reviews (1): CD001044. doi:10.1002/14651858.CD001044. PMID 11869585.
- ↑ Wilt, Timothy J; Ishani, Areef; Rutks, Indulis; MacDonald, Roderick (2007). "Phytotherapy for benign prostatic hyperplasia". Public Health Nutrition. 3 (4A): 459–72. doi:10.1017/S1368980000000549. PMID 11276294.
- ↑ Keehn; Taylor; Lowe (2016). "Phytotherapy for benign prostatic hyperplasia". Curr. Urol. Rep. 17 (7): 53. doi:10.1007/s11934-016-0609-z. PMID 27180172.
- ↑ Ma, CH; Lin, WL; Lui, SL; Cai, XY; Wong, VT; Ziea, E; Zhang, ZJ (July 2013). "Efficacy and safety of Chinese herbal medicine for benign prostatic hyperplasia: systematic review of randomized controlled trials.". Asian Journal of Andrology. 15 (4): 471–82. doi:10.1038/aja.2012.173. PMC 3739225
 . PMID 23728585.
. PMID 23728585. - ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved 11 November 2009.
- ↑ Vos, Theo; et al. (1 December 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010". The Lancet. 380 (9859): 2163–2196. doi:10.1016/S0140-6736(12)61729-2. PMID 23245607.
- ↑ Verhamme, K; Dieleman, JP; Bleumink, GS; Van Der Lei, J; Sturkenboom, MC; Artibani, W; Begaud, B; Berges, R; et al. (2002). "Incidence and Prevalence of Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia in Primary Care—The Triumph Project". European Urology. 42 (4): 323–8. doi:10.1016/S0302-2838(02)00354-8. PMID 12361895.
- ↑ Fernandes, L; Rio Tinto, H; Pereira, J; Duarte, M; Bilhim, T; Martins Pisco, J (December 2012). "Prostatic arterial embolization: post-procedural follow-up.". Techniques in vascular and interventional radiology. 15 (4): 294–9. doi:10.1053/j.tvir.2012.09.008. PMID 23244727.
Further reading
- Christensen, Tyler L.; Andriole, Gerald L. (February 2009). "Benign Prostatic Hyperplasia: Current Treatment Strategies". Consultant. 49 (2): 115–22.
External links
| Wikimedia Commons has media related to Benign prostatic hyperplasia. |