Baker's yeast
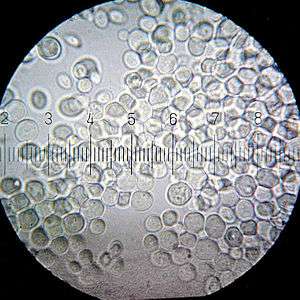
Baker's yeast is the common name for the strains of yeast commonly used as a leavening agent in baking bread and bakery products, where it converts the fermentable sugars present in the dough into carbon dioxide and ethanol. Baker's yeast is of the species Saccharomyces cerevisiae,[1] which is the same species (but a different strain) commonly used in alcoholic fermentation, which is called brewer's yeast.[2] Baker's yeast is also a single-cell microorganism found on and around the human body.
The use of steamed or boiled potatoes,[3] water from potato boiling,[4] or sugar in a bread dough provides food for the growth of yeasts; however, too much sugar will dehydrate them.[5] Yeast growth is inhibited by both salt and sugar, but more so with salt than sugar.[6] Fats, such as butter or eggs, slow down yeast growth;[7] however, others say the effect of fat on dough remains unclear, presenting evidence that small amounts of fat are beneficial for baked bread volume.[8]
Saccharomyces exiguus (also known as S. minor) is a wild yeast found on plants, fruits, and grains that is occasionally used for baking; however, in general it is not used in a pure form but comes from being propagated in a sourdough starter.
History

It is not known when yeast was first used to bake bread; the earliest definite records come from Ancient Egypt.[9] Researchers speculate that a mixture of flour meal and water was left longer than usual on a warm day and the yeasts that occur in natural contaminants of the flour caused it to ferment before baking. The resulting bread would have been lighter and tastier than the previous hard flatbreads. It is generally assumed that the earliest forms of leavening were likely very similar to modern sourdough; the leavening action of yeast would have been discovered from its action on flatbread doughs, and would have been either cultivated separately or transferred from batch to batch by means of previously mixed ("old") dough. Also, the development of leavened bread seems to have developed in close proximity to the development of beer brewing, and barm from the beer fermentation process can also be used in bread making.
Without an understanding of microbiology, early bakers would have had little ability to directly control yeast cultures, but still kept locally interesting cultures by reusing doughs and starters to leaven later batches. However, it became possible to isolate and propagate favored yeast strains in the same manner as was done in the beer industry, and it eventually became practical to propagate yeast in a slurry with a composition similar to beer wort, usually including malted barley and wheat flour. Such cultures (sometimes referred to in old American cookery as "emptins", from their origins as the dregs of beer or cider fermentation) would become the ancestors of modern baker's yeast, as, in general, they were carefully maintained to avoid what would later be discovered to be bacterial contamination, including using preservatives such as hops as well as boiling the growth medium.
In the 19th century, bread bakers obtained their yeast from beer brewers, and this led to sweet-fermented breads such as the Imperial "Kaiser-Semmel" roll,[10] which in general lacked the sourness created by the acidification typical of Lactobacillus. However, beer brewers slowly switched from top-fermenting to bottom-fermenting yeast (both S. cerevisiae) and this created a shortage of yeast for making bread, so the Vienna Process was developed in 1846.[11] While the innovation is often popularly credited for using steam in baking ovens, leading to a different crust characteristic, it is notable for including procedures for high milling of grains (see Vienna grits[12]), cracking them incrementally instead of mashing them with one pass; as well as better processes for growing and harvesting top-fermenting yeasts, known as press-yeast.
Refinements in microbiology following the work of Louis Pasteur led to more advanced methods of culturing pure strains. In 1879, Great Britain introduced specialized growing vats for the production of S. cerevisiae, and in the United States around the turn of the century centrifuges were used for concentrating the yeast,[13] making modern commercial yeast possible, and turning yeast production into a major industrial endeavor. The slurry yeast made by small bakers and grocery shops became cream yeast, a suspension of live yeast cells in growth medium, and then compressed yeast, the fresh cake yeast that became the standard leaven for bread bakers in much of the Westernized world during the early 20th century.
During World War II, Fleischmann's developed a granulated active dry yeast for the United States armed forces, which did not require refrigeration and had a longer shelf-life and better temperature tolerance than fresh yeast; it is still the standard yeast for US military recipes. The company created yeast that would rise twice as fast, cutting down on baking time. Lesaffre would later create instant yeast in the 1970s, which has gained considerable use and market share at the expense of both fresh and dry yeast in their various applications.
Types of baker's yeast
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
| Energy | 1,361 kJ (325 kcal) |
|
41.22 g | |
| Sugars | 0 g |
| Dietary fiber | 26.9 g |
|
7.61 g | |
|
40.44 g | |
| Vitamins | |
| Thiamine (B1) |
(956%) 10.99 mg |
| Riboflavin (B2) |
(333%) 4 mg |
| Niacin (B3) |
(268%) 40.2 mg |
| Pantothenic acid (B5) |
(270%) 13.5 mg |
| Vitamin B6 |
(115%) 1.5 mg |
| Folate (B9) |
(585%) 2340 μg |
| Choline |
(7%) 32 mg |
| Vitamin C |
(0%) 0.3 mg |
| Minerals | |
| Calcium |
(3%) 30 mg |
| Iron |
(17%) 2.17 mg |
| Magnesium |
(15%) 54 mg |
| Manganese |
(15%) 0.312 mg |
| Phosphorus |
(91%) 637 mg |
| Potassium |
(20%) 955 mg |
| Sodium |
(3%) 51 mg |
| Zinc |
(84%) 7.94 mg |
| Other constituents | |
| Water | 5.08 g |
|
| |
| |
|
Percentages are roughly approximated using US recommendations for adults. Source: USDA Nutrient Database | |

Baker's yeast is available in a number of different forms, the main differences being the moisture contents.[14] Though each version has certain advantages over the others, the choice of which form to use is largely a question of the requirements of the recipe at hand and the training of the cook preparing it. Dry yeast forms are good choices for longer-term storage, often lasting more than a year at room temperatures without significant loss of viability.[15] In general, with occasional allowances for liquid content and temperature, the different forms of commercial yeast are considered interchangeable.
- Cream yeast is the closest form to the yeast slurries of the 19th century, in essence being a suspension of yeast cells in liquid, siphoned off from the growth medium. Its primary use is in industrial bakeries with special high-volume dispensing and mixing equipment, and it is not readily available to small bakeries or home cooks.[16]
- Compressed yeast is, in essence, cream yeast with most of the liquid removed. It is a soft solid, beige in color, and best known in the consumer form as small, foil-wrapped cubes of cake yeast. It is also available in larger-block form for bulk usage.[17] It is highly perishable; though formerly widely available for the consumer market, it has become less common in supermarkets in some countries due to its poor keeping properties, having been superseded in some such markets by active dry and instant yeast. It is still widely available for commercial use, and is somewhat more tolerant of low temperatures than other forms of commercial yeast; however, even there, instant yeast has made significant market inroads.
- Active dry yeast is the form of yeast most commonly available to non-commercial bakers in the United States. It consists of coarse oblong granules of yeast, with live yeast cells encapsulated in a thick jacket of dry, dead cells with some growth medium. Under most conditions, active dry yeast must first be proofed or rehydrated. It can be stored at room temperature for a year, or frozen for more than a decade, which means that it has better keeping qualities than other forms, but it is generally considered more sensitive than other forms to thermal shock when actually used in recipes.

- Instant yeast appears similar to active dry yeast, but has smaller granules with substantially higher percentages of live cells per comparable unit volumes.[16] It is more perishable than active dry yeast but also does not require rehydration, and can usually be added directly to all but the driest doughs. In general, instant yeast has a small amount of ascorbic acid added as a preservative. Some producers provide two or more forms of instant yeast in their product portfolio; for example, LeSaffre's "SAF Instant Gold" is designed specifically for doughs with high sugar contents, and such yeasts are more generally known as osmotolerant yeasts.[18]
- Rapid-rise yeast is a variety of dried yeast (usually a form of instant yeast) that is of a smaller granular size, thus it dissolves faster in dough, and it provides greater carbon dioxide output to allow faster rising.[19] There is considerable debate as to the value of such a product; while most baking experts believe it reduces the flavor potential of the finished product, Cook's Illustrated magazine, among others, feels that, at least for direct-rise recipes, it makes little difference. Rapid-rise yeast is often marketed specifically for use in bread machines.
- Deactivated yeast is dead yeast which has no leavening value and is not interchangeable with other yeast types. Typically used for pizza and pan bread doughs, it is used at a rate of 0.1% of the flour weight, though manufacturer specifications may vary. It is a powerful reducing agent used to increase the extensibility of a dough.[20]
For most commercial uses, yeast of any form is packaged in bulk (blocks or freezer bags for fresh yeast; vacuum-packed brick bags for dry or instant); however, yeast for home use is often packaged in pre-measured doses, either small squares for compressed yeast or sealed packets for dry or instant. For active dry and instant yeast, in general a single dose (reckoned for the average bread recipe of between 500 g and 1000 g of dough) is about 2.5 tsp (~12 mL) or about 7 g (1⁄4 oz), though comparatively lesser amounts are used when the yeast is used in a pre-ferment. In general, a yeast flavor in the baked bread is not noticeable when the bakers' percent of added yeast is less than 2.5%.[21]
Use in research
Model organism
Because it is readily available and easy to culture, baker's yeast has long been used in chemical, biological, and genetic research as a model organism. In 1996, after 6 years of work, S. cerevisiae became the first eukaryote to have its entire genome sequenced. It has over 12 million base pairs and around 6000 genes. Since then, it has remained in the forefront of genetic research. For example, most of our knowledge of the cell division cycle was worked out from experiments with yeast.
Organic synthesis
Baker's yeast contains enzymes that can reduce a carbonyl group into a hydroxyl group in fairly high yield, thus making it useful for biotransformations in organic syntheses.[22] It is known to reduce organometallic carbonyl compounds in very high yield.[23]
Baker's yeast can also be used to produce ethanol via fermentation for use in chemical synthesis, although doing so in some places requires permits.
Industrial production
The baking industry relies on industrial production of its ingredients, including baking yeasts. Much effort has been put into developing and marketing yeasts that will perform reliably in mass production. Since the end of the nineteenth century, baker’s yeast has been produced by companies that specialize in its production.
In 2012 the companies that dominated the US market included: Lesaffre Group, AB Vista, DSM, GB Plange and AB Mauri, producing hundreds of thousands of metric tons of yeast each year in industrial plants.
See also
| Wikimedia Commons has media related to Baker's yeast. |
Further reading
- Corriher, Shirley, Cookwise. New York: William Morrow and Co., 1997, ISBN 0-688-10229-8.
- Editors of Cook's Illustrated Magazine, Baking Illustrated. Brookline, MA:Boston Common Press, 2004, ISBN 0-936184-75-2.
- The King Arthur Flour Baker's Companion. Woodstock, VT: Countryman Press, 2003, ISBN 0-88150-581-1.
- Simmons, Amelia. American Cookery, Hartford, 1798. Text at Feeding America and Project Gutenberg.
- Sloat, Caroline (ed.), Old Sturbridge Village Cookbook 2ed.. Old Saybrook: Globe Pequot Press, 1995, ISBN 1564407284.
References
- ↑ Young, Linda; Cauvain, Stanley P. (2007). Technology of Breadmaking. Berlin: Springer. p. 79. ISBN 0-387-38563-0.
The scientific name for baker's yeast is Saccharomyces cerevisiae, ...
- ↑ Kalmus, Sage. "What Is the Difference Between Brewer's Yeast & Baker's Yeast?". livestrong.com. Retrieved 14 May 2013.
- ↑ Eben Norton Horsford (1875). Report on Vienna bread - Google Books. Washington: Government Printing Office. pp. 90, 88, 87.
- ↑ Samuel P. Sadtler (1908). A hand-book of industrial organic ... - Google Books. J. B. Lippincott Company. p. 235.
- ↑ Christian, Elizabeth W.; Vaclavik, Vickie (2003). Essentials of food science. New York: Kluwer Academic/Plenum Publishers. p. 323. ISBN 0-306-47363-1.
- ↑ Young, Linda; Cauvain, Stanley P. (2007). Technology of Breadmaking. Berlin: Springer. p. 88. ISBN 0-387-38563-0.
- ↑ From the Editors of Good Housekeeping; Susan Westmoreland (2004). The Good Housekeeping Cookbook. New York: Hearst. p. 584. ISBN 1-58816-398-9.
- ↑ Young, Linda; Cauvain, Stanley P. (2007). Technology of Breadmaking. Berlin: Springer. p. 54. ISBN 0-387-38563-0. Retrieved 2011-04-25.
- ↑ "The History of Bread Yeast". BBC. Retrieved December 24, 2006.
- ↑ Eben Norton Horsford (1875). Report on Vienna bread - Google Books. Washington: Government Printing Office. p. 86.
- ↑ Kristiansen, B.; Ratledge, Colin (2001). Basic biotechnology. Cambridge, UK: Cambridge University Press. p. 378. ISBN 0-521-77917-0.
- ↑ Eben Norton Horsford (1875). Report on Vienna bread - Google Books. Washington: Government Printing Office. pp. 31–32.
- ↑ Marx, Jean & Litchfield, John H. (1989). A Revolution in biotechnology. Cambridge, UK: Cambridge University Press. p. 71. ISBN 0-521-32749-0.
- ↑ Young, Linda; Cauvain, Stanley P. (2007). Technology of Breadmaking. Berlin: Springer. p. 77. ISBN 0-387-38563-0.
- ↑ EBBUTT LI (May 1961). "The relationship between activity and cell-wall permeability in dried baker's yeast". J. Gen. Microbiol. 25 (1): 87–95. doi:10.1099/00221287-25-1-87. PMID 13725540.
- 1 2 Reinhart, Peter (2001). The bread baker's apprentice: mastering the art of extraordinary bread. Berkeley, Calif: Ten Speed Press. p. 61. ISBN 1-58008-268-8.
- ↑ Gisslen, Wayne (2008). Professional baking. New York: John Wiley. ISBN 0-471-78349-8.
- ↑ Panchal, Chandra J. (1990). Yeast strain selection. New York: M. Dekker. pp. 140–182. ISBN 0-8247-8276-3. Retrieved 2011-04-24.
- ↑ Kay Pastorius (1997). Cruising Cuisine: Fresh Food from the Galley. International Marine/Ragged Mountain Press. p. 184. ISBN 0-07-048703-0.
- ↑ "San Francisco Baking Institute Newsletter" (pdf). 2003. Retrieved 2015-01-12.
- ↑ Cauvain, Stanley P. (2003). Bread making: improving quality. Boca Raton: CRC Press. p. 475. ISBN 1-85573-553-9.
- ↑ Csuk, Rene.; Glaenzer, Brigitte I. (1991-01-01). "Baker's yeast mediated transformations in organic chemistry". Chemical Reviews. 91 (1): 49–97. doi:10.1021/cr00001a004. ISSN 0009-2665.
- ↑ Paquette, Leo A. (1999). Handbook of Reagents for Organic Synthesis: Chiral Reagents for Asymmetric Synthesis (1st ed.). New York: Wiley. p. 45. ISBN 9780470856253.
