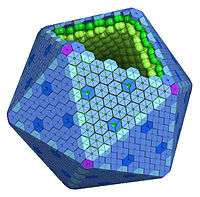
Bacterial microcompartment
]
Bacterial microcompartments (BMCs) are organelles consisting of a protein shell that encloses enzymes and other proteins. BMCs are typically about 40–200 nanometers in diameter and are entirely made of proteins.[1][2][3][4][5][6][7] The shell functions like a membrane, as it is selectively permeable.[2][4][6][8][9] Other protein-based compartments found in bacteria and archaea include encapsulin nanocompartments[10] and gas vesicles.[11] Eukaryotes have also been observed to have proteinaceous organelles, such as the mysterious vault complex.
Discovery
The first BMCs were observed in the 1950s in electron micrographs of cyanobacteria,[12] and were later named carboxysomes after their role in carbon fixation was established.[13] Until the 1990s, carboxysomes were thought to be an oddity confined to certain autotrophic bacteria. But then genes coding for proteins homologous to those of the carboxysome shell were identified in the pdu (propanediol utilization)[14] and eut (ethanolamine utilization)[15] operons. Subsequently, transmission electron micrographs of Salmonella cells grown on propanediol [16] or ethanolamine [17] showed the presence of polyhedral bodies similar to carboxysomes. The term metabolosome is used to refer to such catabolic BMCs (in contrast to the autotrophic carboxysome).
Although the carboxysome, propanediol utilizing (PDU), and ethanolamine utilizing (EUT) BMCs encapsulate different enzymes and therefore have different functions, the genes encoding for the shell proteins are very similar. Most of the genes (coding for the shell proteins and the encapsulated enzymes) from experimentally characterized BMCs are located near one another in distinct genetic loci or operons. There are currently over 20,000 bacterial genomes sequenced, and bioinformatics methods can be used to find all BMC shell genes and to look at what other genes are in the vicinity, producing a list of potential BMCs.[6][18][19] Recently a comprehensive survey identified 23 different loci encoding up to 10 functionally distinct BMCs across 23 bacterial phyla.[19]
Shells
Protein families forming the shell
The BMC shell appears icosahedral or quasi-icosahedral, and is formed by (pseudo)hexameric and pentameric protein subunits.

The BMC shell protein family
The major constituents of the BMC shell are proteins containing Pfam00936 domain(s). These proteins form oligomers that are hexagonal in shape and are thought to form the facets of the shell.[2][20][21]
Single-domain proteins (BMC-H)
The BMC-H proteins, which contain a single copy of the Pfam00936 domain, are the most abundant component of the facets of the shell. The crystal structures of a number of these proteins have been determined, showing that they assemble into cyclical hexamers, typically with a small pore in the center.[2] This opening is proposed to be involved in the selective transport of the small metabolites across the shell.
Tandem-domain proteins (BMC-T)
A subset of shell proteins are composed of tandem (fused) copies of the Pfam00936 domain (BMC-T proteins). The structurally characterized BMC-T proteins form trimers that are pseudohexameric in shape.[22][23][24] Some BMC-T crystal structures show that the trimers can stack in a face-to-face fashion. In such structures, one pore from one trimer is in an “open” conformation, while the other is closed – suggesting that there may be an airlock-like mechanism that modulates the permeability of some BMC shells.[22][25] Another subset of BMC-T proteins contain a [4Fe-4S] cluster, and may be involved in electron transport across the BMC shell.[26][27][28][29][30]
The EutN/CcmL family (BMC-P)
Twelve pentagonal units are necessary to cap the vertices of an icosahedral shell. Crystal structures of proteins from the EutN/CcmL family (Pfam03319) have been solved and they typically form pentamers (BMC-P).[31][32][33] The importance of the BMC-P proteins in shell formation seems to vary among the different BMCs. It was shown that they are necessary for the formation of the shell of the PDU BMC as mutants in which the gene for the BMC-P protein was deleted cannot form shells,[34] but not for the alpha-carboxysome: without BMC-P proteins, carboxysomes will still assemble and many are elongated; these mutant carboxysomes appear to be “leaky”.[35]
Relation to viral capsids
While the BMC shell is architecturally similar to many viral capsids, the shell proteins have not been found to have any structural or sequence homology to capsid proteins.
Permeability of the shell
It is well established that enzymes are packaged within the BMC shell and that some degree of metabolite and cofactor sequestration must occur.[4] However, other metabolites and cofactors must also be allowed to cross the shell in order for BMCs to function. For example, in carboxysomes, ribulose-1,5-bisphosphate, bicarbonate, and phosphoglycerate must cross the shell, while carbon dioxide and oxygen diffusion is apparently limited.[36][37] Similarly, for the PDU BMC, the shell must be permeable to propanediol, propanol, propionyl-phosphate, and potentially also vitamin B12, but it is clear that propionaldehyde is somehow sequestered to prevent cell damage.[38] There is some evidence that ATP must also cross some BMC shells.[4]
It has been proposed that the central pore formed in the hexagonal protein tiles of the shell are the conduits through which metabolites diffuse into the shell.[2][20] For example, the pores in the carboxysome shell have an overall positive charge, which has been proposed to attract negatively charged substrates such as bicarbonate.[2][4][9][20] In the PDU microcompartment, mutagenesis experiments have shown that the pore of the PduA shell protein is the route for entry of the propanediol substrate.[39] For larger metabolites, a gating mechanism in some BMC-T proteins is apparent.[22][25][40] In the EUT microcompartment, gating of the large pore in the EutL shell protein is regulated by the presence of the main metabolic substrate, ethanolamine.[41]
The presence of iron-sulfur clusters in some shell proteins, presumably in the central pore, has led to the suggestion that they can serve as a conduit through which electrons can be shuttled across the shell.[26][29][30]
Types
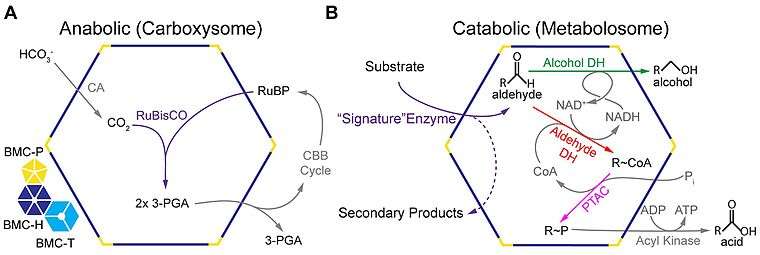
A recent comprehensive survey of microbial genome sequence data indicated up to ten different metabolic functions encapsulated by BMC shells.[19] The majority are involved in either carbon fixation (carboxysomes) or aldehyde oxidation (metabolosomes).[19]
Carboxysomes: carbon fixation

Carboxysomes encapsulate ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) and carbonic anhydrase in carbon-fixing bacteria as part of a carbon concentrating mechanism.[42] Bicarbonate is pumped into the cytosol and diffuses into the carboxysome, where carbonic anhydrase converts it to carbon dioxide, the substrate of RuBisCO. The carboxysome shell is thought to be only sparingly permeable to carbon dioxide, which results in an effective increase in carbon dioxide concentration around RuBisCO, thus enhancing carbon fixation.[37][43] Mutants that lack genes coding for the carboxysome shell display a high carbon requiring phenotype due to the loss of the concentration of carbon dioxide, resulting in increased oxygen fixation by RuBisCO. The shells have also been proposed to restrict the diffusion of oxygen,[9][37] thus preventing the oxygenase reaction, reducing wasteful photorespiration.[36]

Metabolosomes: aldehyde oxidation
In addition to the anabolic carboxysomes, several catabolic BMCs have been characterized that participate in the heterotrophic metabolism via short-chain aldehydes; they are collectively termed metabolosomes.[4][17]
These BMCs share a common encapsulated chemistry driven by three core enzymes: aldehyde dehydrogenase, alcohol dehydrogenase, and phosphotransacylase.[4][19][44] Because aldehydes can be toxic to cells[38] and/or volatile,[45] they are thought to be sequestered within the metabolosome. The aldehyde is initially fixed to coenzyme A by a NAD+-dependent aldehyde dehydrogenase, but these two cofactors must be recycled, as they apparently cannot cross the shell.[46][47] These recycling reactions are catalyzed by an alcohol dehydrogenase (NAD+),[46] and a phosphotransacetylase (coenzyme A),[47] resulting in a phosphorylated acyl compound that can readily be a source of substrate-level phosphorylation or enter central metabolism, depending on if the organism is growing aerobically or anaerobically.[38] It seems that most, if not all, metabolosomes utilize these core enzymes. Metabolosomes also encapsulate another enzyme that is specific to the initial substrate of the BMC, that generates the aldehyde; this is considered the signature enzyme of the BMC.[4][19]
PDU BMCs

Some bacteria can use 1,2-propanediol as a carbon source. They use a BMC to encapsulate several enzymes used in this pathway (Sampson and Bobik, 2008). The PDU BMC is typically encoded by a 21 gene locus. These genes are sufficient for assembly of the BMC since they can be transplanted from one type of bacterium to another, resulting in a functional metabolosome in the recipient.[28] This is an example of bioengineering that likewise provides evidence in support of the selfish operon hypothesis.[48] 1,2-propanediol is dehydrated to propionaldehyde by propanediol dehydratase, which requires vitamin B12 as a cofactor.[49] Propionaldehyde causes DNA mutations and as a result is toxic to cells, possibly explaining why this compound is sequestered within a BMC.[38] The end-products of the PDU BMC are propanol and propionyl-phosphate, which is then dephosphorylated to propionate, generating one ATP. Propanol and propionate can be used as substrates for growth.[38]
EUT BMCs
Ethanolamine utilization (EUT) BMCs are encoded in many diverse types of bacteria.[19] Ethanolamine is cleaved to ammonia and acetaldehyde through the action of ethanolamine-ammonia lyase, which also requires vitamin B12 as a cofactor.[50] Acetaldehyde is fairly volatile, and mutants deficient in the BMC shell have been observed to have a growth defect and release excess amounts of acetaldehyde.[45] It has been proposed that sequestration of acetaldehyde in the metabolosome prevents its loss by volatility.[45] The end-products of the EUT BMC are ethanol and acetyl-phosphate. Ethanol is likely a lost carbon source, but acetyl-phosphate can either generate ATP or be recycled to acetyl-CoA and enter the TCA cycle or several biosynthetic pathways.[17]
Bifunctional PDU/EUT BMCs
Some bacteria, especially those in the Listeria genus, encode a single locus in which genes for both PDU and EUT BMCs are present.[19] It is not yet clear whether this is truly a chimeric BMC with a mixture of both sets of proteins, or if two separate BMCs are formed.
Glycyl radical enzyme-containing BMCs (GRM)
Several different BMC loci have been identified that contain glycyl radical enzymes,[18][19] which obtain the catalytic radical from the cleavage of s-adenosylcobalamin.[51] One GRM locus in Clostridium phytofermentans has been shown to be involved in the fermentation of fucose and rhamnose, which are initially degraded to 1,2-propanediol under anaerobic conditions. The glycyl radical enzyme is proposed to dehydrate propanediol to propionaldehyde, which is then processed in a manner identical to the canonical PDU BMC.[52]
Planctomycetes and Verrucomicrobia BMCs (PVM)
Distinct lineages of Planctomycetes and Verrucomicrobia encode a BMC locus. The locus in Planctomyces limnophilus has been shown to be involved in the aerobic degradation of fucose and rhamnose. An aldolase is thought to generate lactaldehyde, which is then processed through the BMC, resulting in 1,2-propanediol and lactyl-phosphate.[44]
Rhodococcus and Mycobacterium BMCs (RMM)
Two types of BMC loci have been observed in members of the Rhodococcus and Mycobacterium genera, although their actual function has not been established.[19] However, based on the characterized function of one of the genes present in the locus and the predicted functions of the other genes, it was proposed that these loci could be involved in the degradation of amino-2-propanol. The aldehyde generated in this predicted pathway would be the extremely toxic compound methylglyoxal; its sequestration within the BMC could protect the cell.[19]
BMCs of unknown function (BUF)
One type of BMC locus does not contain RuBisCO or any of the core metabolosome enzymes, and has been proposed to facilitate a third category of biochemical transformations (i.e. not carbon fixation or aldehyde oxidation).[19] The presence of genes predicted to code for amidohydrolases and deaminases could indicate that this BMC is involved in the metabolism of nitrogenous compounds.[19]
Assembly
Carboxysomes
The assembly pathway for beta-carboxysomes has been identified, and begins with the protein CcmM nucleating RuBisCO.[53] CcmM has two domains: an N-terminal gamma-carbonic anhydrase domain followed by a domain consisting of three to five repeats of RuBisCO small-subunit-like sequences.[54] The C-terminal domain aggregates RuBisCO, likely by substituting for the actual RuBisCO small subunits in the L8-S8 holoenzyme, effectively cross-linking the RuBisCO in the cell into one large aggregate, termed the procarboxysome.[53] The N-terminal domain of CcmM physically interacts with the N-terminal domain of the CcmN protein, which, in turn, recruits the hexagonal shell protein subunits via an encapsulation peptide on its C-terminus.[55] Carboxysomes are then spatially aligned in the cyanobacterial cell via interaction with the bacterial cytoskeleton, ensuring their equal distribution into daughter cells.[56]
Alpha-carboxysome assembly may be different than that of beta-carboxysomes,[57] as they have no proteins homologous to CcmN or CcmM and no encapsulation peptides. Empty carboxysomes have been observed in electron micrographs.[58] Some micrographs indicate that their assembly occurs as a simultaneous coalescence of enzymes and shell proteins as opposed to the seemingly stepwise fashion observed for beta-carboxysomes.
Metabolosomes
Metabolosome assembly is likely similar to that of the beta-carboxysome,[4][53] via an initial aggregation of the proteins to be encapsulated. The core proteins of many metabolosomes aggregate when expressed alone.[59][60][61][62] Moreover, many encapsulated proteins contain terminal extensions that are strikingly similar to the C-terminal peptide of CcmN that recruits shell proteins.[55][63] These encapsulation peptides are short (about 18 residues) and are predicted to form amphipathic alpha-helices.[55] Some of these helices have been shown to mediate the encapsulation of native enzymes into BMCs, as well as heterologous proteins (such as GFP).[55][64][65][66][67]
Regulation (genetic)
With the exception of carboxysomes, in all tested cases, BMCs are encoded in operons that are expressed only in the presence of their substrate.
PDU BMCs in Salmonella enterica are induced by the presence of propanediol or glycerol under anaerobic conditions, and only propanediol under aerobic conditions.[68] This induction is mediated by the global regulator proteins Crp and ArcA (sensing cyclic AMP and anaerobic conditions respectively),[69] and the regulatory protein PocR, which is the transcriptional activator for both the pdu and the cob loci (the operon necessary for the synthesis of vitamin B12, a required cofactor for propanediol dehydratase).[68]
EUT BMCs in Salmonella enterica are induced via the regulatory protein EutR by the simultaneous presence of ethanolamine and vitamin B12, which can happen under aerobic or anaerobic conditions. Salmonella enterica can only produce endogenous vitamin B12 under anaerobic conditions, although it can import cyanobalamin and convert it to vitamin B12 under either aerobic or anaerobic conditions.[70]
PVM BMCs in Planctomyces limnophilus are induced by the presence of fucose or rhamnose under aerobic conditions, but not by glucose.[44] Similar results were obtained for the GRM BMC from Clostridium phytofermentans, for which both sugars induce the genes coding for the BMC as well as the ones coding for fucose and rhamnose dissimilatory enzymes.[52]
In addition to characterized regulatory systems, bioinformatics surveys have indicated that there are potentially many other regulatory mechanisms, even within a functional type of BMC (e.g. PDU), including two-component regulatory systems.[19]
Relevance to global and human health
Carboxysomes are present in all cyanobacteria and many other photo- and chemoautotrophic bacteria. Cyanobacteria are globally significant drivers of carbon fixation, and since they require carboxysomes to do so in current atmospheric conditions, the carboxysome is a major component of global carbon dioxide fixation.
Several types of BMCs have been implicated in virulence of pathogens, such as Salmonella enterica and Listeria monocytogenes. BMC genes tend to be upregulated under virulence conditions, and mutating them leads to a virulence defect as judged by competition experiments.[71][72][73][74][75]
Biotechnological applications
Several features of BMCs make them appealing for biotechnological applications. Because carboxysomes increase the efficiency of carbon fixation, much research effort has gone into introducing carboxysomes in plants in order to improve the carbon concentrating mechanism.[76][77]
More generally, because BMC shell proteins self-assemble, empty shells can be formed,[34][67] prompting efforts to engineer them to contain customized cargo. Discovery of the encapsulation peptide on the termini of some BMC-associated proteins[55][64] provides a means to begin to engineer custom BMCs by fusing foreign proteins to this peptide and co-expressing this with shell proteins. For example, by adding this peptide to pyruvate decarboxylase and alcohol dehydrogenase, researchers have engineered an ethanol bioreactor.[78] Finally, the pores present in the shell proteins control the permeability of the shell: these can be a target for bioengineering, as they can be modified to allow the crossing of selected substrates and products.[79]
See also
- Endomembrane system
- Metabolic pathway
- Substrate channeling
- Encapsulins
References
- ↑ Cheng, Shouqiang; Liu, Yu; Crowley, Christopher S.; Yeates, Todd O.; Bobik, Thomas A. (2008). "Bacterial microcompartments: their properties and paradoxes". BioEssays. 30 (11-12): 1084–1095. doi:10.1002/bies.20830. ISSN 0265-9247.
- 1 2 3 4 5 6 Kerfeld CA, Sawaya MR, Tanaka S, Nguyen CV, Phillips M, Beeby M, Yeates TO (August 2005). "Protein structures forming the shell of primitive bacterial organelles". Science. 309 (5736): 936–938. doi:10.1126/science.1113397. PMID 16081736.
- ↑ Yeates, Todd O.; Kerfeld, Cheryl A.; Heinhorst, Sabine; Cannon, Gordon C.; Shively, Jessup M. (2008). "Protein-based organelles in bacteria: carboxysomes and related microcompartments". Nature Reviews Microbiology. 6 (9): 681–691. doi:10.1038/nrmicro1913. ISSN 1740-1526. PMID 18679172.
- 1 2 3 4 5 6 7 8 9 Kerfeld, Cheryl A.; Erbilgin, Onur (2015). "Bacterial microcompartments and the modular construction of microbial metabolism". Trends in Microbiology. 23 (1): 22–34. doi:10.1016/j.tim.2014.10.003. ISSN 0966-842X.
- ↑ Cannon GC, Bradburne CE, Aldrich HC, Baker SH, Heinhorst S, Shively JM (December 2001). "Microcompartments in prokaryotes: carboxysomes and related polyhedra". Applied and Environmental Microbiology. 67 (12): 5351–5361. doi:10.1128/AEM.67.12.5351-5361.2001. PMC 93316
 . PMID 11722879.
. PMID 11722879. - 1 2 3 Kerfeld, Cheryl A.; Heinhorst, Sabine; Cannon, Gordon C. (2010). "Bacterial Microcompartments". Annual Review of Microbiology. 64 (1): 391–408. doi:10.1146/annurev.micro.112408.134211. ISSN 0066-4227.
- ↑ Yeates, Todd O.; Crowley, Christopher S.; Tanaka, Shiho (2010). "Bacterial Microcompartment Organelles: Protein Shell Structure and Evolution". Annu. Rev. Biophys. 39: 185–205. doi:10.1146/annurev.biophys.093008.131418. PMC 3272493
 . PMID 20192762.
. PMID 20192762. - ↑ Yeates, Todd O.; Thompson, Michael C.; Bobik, Thomas A. (2011). "The Protein Shells of Bacterial Microcompartment Organelles". Curr. Opin. Struct. Biol. 21 (2): 223–231. doi:10.1016/j.sbi.2011.01.006. PMC 3070793
 . PMID 21315581.
. PMID 21315581. - 1 2 3 Kinney, James N.; Axen, Seth D.; Kerfeld, Cheryl A. (2011). "Comparative analysis of carboxysome shell proteins". Photosynthesis Research. 109 (1-3): 21–32. doi:10.1007/s11120-011-9624-6. ISSN 0166-8595.
- ↑ Sutter, Markus; Boehringer, Daniel; Gutmann, Sascha; Günther, Susanne; Prangishvili, David; Loessner, Martin J; Stetter, Karl O; Weber-Ban, Eilika; Ban, Nenad (2008). "Structural basis of enzyme encapsulation into a bacterial nanocompartment". Nature Structural & Molecular Biology. 15 (9): 939–947. doi:10.1038/nsmb.1473. ISSN 1545-9993.
- ↑ Pfeifer, Felicitas (2012). "Distribution, formation and regulation of gas vesicles". Nature Reviews Microbiology. 10 (10): 705–715. doi:10.1038/nrmicro2834. ISSN 1740-1526.
- ↑ G. DREWS & W. NIKLOWITZ (1956). "[Cytology of Cyanophycea. II. Centroplasm and granular inclusions of Phormidium uncinatum]". Archiv für Mikrobiologie. 24 (2): 147–162. PMID 13327992.
- ↑ Shively JM, Ball F, Brown DH, Saunders RE (November 1973). "Functional organelles in prokaryotes: polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus". Science. 182 (4112): 584–586. doi:10.1126/science.182.4112.584. PMID 4355679.
- ↑ P. Chen, D. I. Andersson & J. R. Roth (September 1994). "The control region of the pdu/cob regulon in Salmonella typhimurium". Journal of Bacteriology. 176 (17): 5474–5482. PMID 8071226.
- ↑ I. Stojiljkovic, A. J. Baumler & F. Heffron (March 1995). "Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster". Journal of Bacteriology. 177 (5): 1357–1366. PMID 7868611.
- ↑ Bobik TA, Havemann GD, Busch RJ, Williams DS, Aldrich HC (October 1999). "The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B(12)-dependent 1, 2-propanediol degradation". Journal of Bacteriology. 181 (19): 5967–5975. PMID 10498708.
- 1 2 3 Brinsmade, S. R.; Paldon, T.; Escalante-Semerena, J. C. (2005). "Minimal Functions and Physiological Conditions Required for Growth of Salmonella enterica on Ethanolamine in the Absence of the Metabolosome". Journal of Bacteriology. 187 (23): 8039–8046. doi:10.1128/JB.187.23.8039-8046.2005. ISSN 0021-9193.
- 1 2 Jorda, Julien; Lopez, David; Wheatley, Nicole M.; Yeates, Todd O. (2013). "Using comparative genomics to uncover new kinds of protein-based metabolic organelles in bacteria". Protein Science. 22 (2): 179–195. doi:10.1002/pro.2196. ISSN 0961-8368.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Axen, Seth D.; Erbilgin, Onur; Kerfeld, Cheryl A. (2014). "A Taxonomy of Bacterial Microcompartment Loci Constructed by a Novel Scoring Method". PLoS Computational Biology. 10 (10): e1003898. doi:10.1371/journal.pcbi.1003898. ISSN 1553-7358.
- 1 2 3 4 Tsai Y, Sawaya MR, Cannon GC, Cai F, Williams EB, Heinhorst S, Kerfeld CA, Yeates TO (June 2007). "Structural analysis of CsoS1A and the protein shell of the Halothiobacillus neapolitanus carboxysome". PLoS Biology. 5 (6): e144. doi:10.1371/journal.pbio.0050144. PMC 1872035
 . PMID 17518518.
. PMID 17518518. - ↑ Dryden, K.A.; Crowley, C.S.; Tanaka, S.; Yeates, T.O.; Yeager, M. (2009). "Two-Dimensional Crystals of Carboxysome Shell Proteins Recapitulate the Hexagonal Packing of Three-Dimensional Crystals". Protein Sci. 18 (12): 2629–2635. doi:10.1002/pro.272. PMC 2821281
 . PMID 19844993.
. PMID 19844993. - 1 2 3 Klein, Michael G.; Zwart, Peter; Bagby, Sarah C.; Cai, Fei; Chisholm, Sallie W.; Heinhorst, Sabine; Cannon, Gordon C.; Kerfeld, Cheryl A. (2009). "Identification and Structural Analysis of a Novel Carboxysome Shell Protein with Implications for Metabolite Transport". Journal of Molecular Biology. 392 (2): 319–333. doi:10.1016/j.jmb.2009.03.056. ISSN 0022-2836.
- ↑ Sagermann, M.; Ohtaki, A.; Nikolakakis, K. (2009). "Crystal structure of the EutL shell protein of the ethanolamine ammonia lyase microcompartment". Proceedings of the National Academy of Sciences. 106 (22): 8883–8887. doi:10.1073/pnas.0902324106. ISSN 0027-8424.
- ↑ Heldt, Dana; Frank, Stefanie; Seyedarabi, Arefeh; Ladikis, Dimitrios; Parsons, Joshua B.; Warren, Martin J.; Pickersgill, Richard W. (2009). "Structure of a trimeric bacterial microcompartment shell protein, EtuB, associated with ethanol utilization inClostridium kluyveri". Biochemical Journal. 423 (2): 199–207. doi:10.1042/BJ20090780. ISSN 0264-6021. PMID 19635047.
- 1 2 Cai, F.; Sutter, M.; Cameron, J. C.; Stanley, D. N.; Kinney, J. N.; Kerfeld, C. A. (2013). "The Structure of CcmP, a Tandem Bacterial Microcompartment Domain Protein from the -Carboxysome, Forms a Subcompartment Within a Microcompartment". Journal of Biological Chemistry. 288 (22): 16055–16063. doi:10.1074/jbc.M113.456897. ISSN 0021-9258.
- 1 2 Crowley, Christopher S.; Cascio, Duilio; Sawaya, Michael R.; Kopstein, Jefferey S.; Bobik, Thomas A.; Yeates, Todd O. (2010). "Structural Insight into the Mechanisms of Transport Across the Salmonella Enterica Pdu Microcompartment Shell". Journal of Biological Chemistry. 285 (48): 37838–37846. doi:10.1074/jbc.M110.160580.
- ↑ Pang, Allan; Warren, Martin J.; Pickersgill, Richard W. (2011). "Structure of PduT, a trimeric bacterial microcompartment protein with a 4Fe–4S cluster-binding site". Acta Crystallographica Section D. 67 (2): 91–96. doi:10.1107/S0907444910050201. ISSN 0907-4449. PMID 21245529.
- 1 2 Parsons, J. B.; Dinesh, S. D.; Deery, E.; Leech, H. K.; Brindley, A. A.; Heldt, D.; Frank, S.; Smales, C. M.; Lunsdorf, H.; Rambach, A.; Gass, M. H.; Bleloch, A.; McClean, K. J.; Munro, A. W.; Rigby, S. E. J.; Warren, M. J.; Prentice, M. B. (2008). "Biochemical and Structural Insights into Bacterial Organelle Form and Biogenesis". Journal of Biological Chemistry. 283 (21): 14366–14375. doi:10.1074/jbc.M709214200. ISSN 0021-9258. PMID 18332146.
- 1 2 Parsons, Joshua B.; Lawrence, Andrew D.; McLean, Kirsty J.; Munro, Andrew W.; Rigby, Stephen E. J.; Warren, Martin J. (2010). "Characterisation of PduS, the pdu Metabolosome Corrin Reductase, and Evidence of Substructural Organisation within the Bacterial Microcompartment". PLoS ONE. 5 (11): e14009. doi:10.1371/journal.pone.0014009. ISSN 1932-6203.
- 1 2 Thompson, Michael C.; Wheatley, Nicole M.; Jorda, Julien; Sawaya, Michael R.; Gidaniyan, Soheil; Ahmed, Hoda; Yang, Z; McCarty, Crystal; Whitelegge, Julien; Yeates, Todd O. (2014). "Identification of a Unique Fe-S Cluster Binding Site in a Glycyl-Radical Type Microcompartment Shell Protein". Journal of Molecular Biology. 426 (19): 3287–3304. doi:10.1016/j.jmb.2014.07.018. PMC 4175982
 . PMID 25102080.
. PMID 25102080. - ↑ Tanaka, S.; Kerfeld, C. A.; Sawaya, M. R.; Cai, F.; Heinhorst, S.; Cannon, G. C.; Yeates, T. O. (2008). "Atomic-Level Models of the Bacterial Carboxysome Shell". Science. 319 (5866): 1083–1086. doi:10.1126/science.1151458. ISSN 0036-8075. PMID 18292340.
- ↑ Sutter, Markus; Wilson, Steven C.; Deutsch, Samuel; Kerfeld, Cheryl A. (2013). "Two new high-resolution crystal structures of carboxysome pentamer proteins reveal high structural conservation of CcmL orthologs among distantly related cyanobacterial species". Photosynthesis Research. 118 (1-2): 9–16. doi:10.1007/s11120-013-9909-z. ISSN 0166-8595.
- ↑ Wheatley, Nicole M.; Gidaniyan, Soheil D.; Liu, Yuxi; Cascio, Duilio; Yeates, Todd O. (2013). "Bacterial microcompartment shells of diverse functional types possess pentameric vertex proteins". Protein Science. 22 (5): 660–665. doi:10.1002/pro.2246. ISSN 0961-8368.
- 1 2 Parsons, Joshua B.; Frank, Stefanie; Bhella, David; Liang, Mingzhi; Prentice, Michael B.; Mulvihill, Daniel P.; Warren, Martin J. (2010). "Synthesis of Empty Bacterial Microcompartments, Directed Organelle Protein Incorporation, and Evidence of Filament-Associated Organelle Movement". Molecular Cell. 38 (2): 305–315. doi:10.1016/j.molcel.2010.04.008. ISSN 1097-2765.
- ↑ Cai, Fei; Menon, Balaraj B.; Cannon, Gordon C.; Curry, Kenneth J.; Shively, Jessup M.; Heinhorst, Sabine (2009). "The Pentameric Vertex Proteins Are Necessary for the Icosahedral Carboxysome Shell to Function as a CO2 Leakage Barrier". PLoS ONE. 4 (10): e7521. doi:10.1371/journal.pone.0007521. ISSN 1932-6203.
- 1 2 Marcus, Yehouda; Berry, JosephA.; Pierce, John (1992). "Photosynthesis and photorespiration in a mutant of the cyanobacterium Synechocystis PCC 6803 lacking carboxysomes". Planta. 187 (4). doi:10.1007/BF00199970. ISSN 0032-0935.
- 1 2 3 Dou, Z.; Heinhorst, S.; Williams, E. B.; Murin, C. D.; Shively, J. M.; Cannon, G. C. (2008). "CO2 Fixation Kinetics of Halothiobacillus neapolitanus Mutant Carboxysomes Lacking Carbonic Anhydrase Suggest the Shell Acts as a Diffusional Barrier for CO2". Journal of Biological Chemistry. 283 (16): 10377–10384. doi:10.1074/jbc.M709285200. ISSN 0021-9258.
- 1 2 3 4 5 Sampson, E. M.; Bobik, T. A. (2008). "Microcompartments for B12-Dependent 1,2-Propanediol Degradation Provide Protection from DNA and Cellular Damage by a Reactive Metabolic Intermediate". Journal of Bacteriology. 190 (8): 2966–2971. doi:10.1128/JB.01925-07. ISSN 0021-9193. PMC 2293232
 . PMID 18296526.
. PMID 18296526. - ↑ Chowdhury, C.; Chun, Sunny; Pang, Allan; Sawaya, Michael R.; Sinha, S.; Yeates, Todd O.; Bobik, Thomas A. (2015). "Selective Molecular Transport Through the Protein Shell of a Bacterial Microcompartment Organelle". Proc. Natl. Acad. Sci. U.S.A. 112 (10): 2990–2995. doi:10.1073/pnas.1423672112. PMC 4364225
 . PMID 25713376.
. PMID 25713376. - ↑ Tanaka, Shiho; Sawaya, Michael R.; Yeates, Todd O. (2010). "Structure and Mechanisms of a Protein-Based Organelle in Escherichia coli". Science. 327 (596): 81–84. doi:10.1126/science.1179513. PMID 20044574.
- ↑ Thompson, Michael C.; Cascio, Duilio; Leibly, David J.; Yeates, Todd O. (2015). "An Allosteric Model for Control of Pore Opening by Substrate Binding in the EutL Microcompartment Shell Protein". Protein Science. 24 (6): 956–975. doi:10.1002/pro.2672. PMC 4456109
 . PMID 25752492.
. PMID 25752492. - ↑ Murray R. Badger & G. Dean Price (February 2003). "CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution". Journal of Experimental Botany. 54 (383): 609–622. doi:10.1093/jxb/erg076. PMID 12554704.
- ↑ G. D. Price & M. R. Badger (October 1989). "Expression of Human Carbonic Anhydrase in the Cyanobacterium Synechococcus PCC7942 Creates a High CO(2)-Requiring Phenotype : Evidence for a Central Role for Carboxysomes in the CO(2) Concentrating Mechanism". Plant physiology. 91 (2): 505–513. doi:10.1104/pp.91.2.505. PMID 16667062.
- 1 2 3 Erbilgin, O.; McDonald, K. L.; Kerfeld, C. A. (2014). "Characterization of a Planctomycetal Organelle: a Novel Bacterial Microcompartment for the Aerobic Degradation of Plant Saccharides". Applied and Environmental Microbiology. 80 (7): 2193–2205. doi:10.1128/AEM.03887-13. ISSN 0099-2240.
- 1 2 3 Joseph T. Penrod & John R. Roth (April 2006). "Conserving a volatile metabolite: a role for carboxysome-like organelles in Salmonella enterica". Journal of Bacteriology. 188 (8): 2865–2874. doi:10.1128/JB.188.8.2865-2874.2006. PMC 1447003
 . PMID 16585748.
. PMID 16585748. - 1 2 Cheng, Shouqiang; Fan, Chenguang; Sinha, Sharmistha; Bobik, Thomas A. (2012). "The PduQ Enzyme Is an Alcohol Dehydrogenase Used to Recycle NAD+ Internally within the Pdu Microcompartment of Salmonella enterica". PLoS ONE. 7 (10): e47144. doi:10.1371/journal.pone.0047144. ISSN 1932-6203.
- 1 2 Huseby, D. L.; Roth, J. R. (2013). "Evidence that a Metabolic Microcompartment Contains and Recycles Private Cofactor Pools". Journal of Bacteriology. 195 (12): 2864–2879. doi:10.1128/JB.02179-12. ISSN 0021-9193.
- ↑ J. G. Lawrence & J. R. Roth (August 1996). "Selfish operons: horizontal transfer may drive the evolution of gene clusters". Genetics. 143 (4): 1843–1860. PMC 1207444
 . PMID 8844169.
. PMID 8844169. - ↑ R. M. Jeter (May 1990). "Cobalamin-dependent 1,2-propanediol utilization by Salmonella typhimurium". Journal of General Microbiology. 136 (5): 887–896. doi:10.1099/00221287-136-5-887. PMID 2166132.
- ↑ D. M. Roof & J. R. Roth (June 1989). "Functions required for vitamin B12-dependent ethanolamine utilization in Salmonella typhimurium". Journal of Bacteriology. 171 (6): 3316–3323. PMID 2656649.
- ↑ Frey, Perry A.; Hegeman, Adrian D.; Ruzicka, Frank J. (2008). "The Radical SAM Superfamily". Critical Reviews in Biochemistry and Molecular Biology. 43 (1): 63–88. doi:10.1080/10409230701829169. ISSN 1040-9238. PMID 18307109.
- 1 2 Petit, Elsa; LaTouf, W. Greg; Coppi, Maddalena V.; Warnick, Thomas A.; Currie, Devin; Romashko, Igor; Deshpande, Supriya; Haas, Kelly; Alvelo-Maurosa, Jesús G.; Wardman, Colin; Schnell, Danny J.; Leschine, Susan B.; Blanchard, Jeffrey L. (2013). "Involvement of a Bacterial Microcompartment in the Metabolism of Fucose and Rhamnose by Clostridium phytofermentans". PLoS ONE. 8 (1): e54337. doi:10.1371/journal.pone.0054337. ISSN 1932-6203.
- 1 2 3 Cameron, Jeffrey C.; Wilson, Steven C.; Bernstein, Susan L.; Kerfeld, Cheryl A. (2013). "Biogenesis of a Bacterial Organelle: The Carboxysome Assembly Pathway". Cell. 155 (5): 1131–1140. doi:10.1016/j.cell.2013.10.044. ISSN 0092-8674.
- ↑ Long BM, Badger MR, Whitney SM, Price GD (October 2007). "Analysis of carboxysomes from Synechococcus PCC7942 reveals multiple Rubisco complexes with carboxysomal proteins CcmM and CcaA". The Journal of Biological Chemistry. 282 (40): 29323–29335. doi:10.1074/jbc.M703896200. PMID 17675289.
- 1 2 3 4 5 Kinney, J. N.; Salmeen, A.; Cai, F.; Kerfeld, C. A. (2012). "Elucidating Essential Role of Conserved Carboxysomal Protein CcmN Reveals Common Feature of Bacterial Microcompartment Assembly". Journal of Biological Chemistry. 287 (21): 17729–17736. doi:10.1074/jbc.M112.355305. ISSN 0021-9258.
- ↑ Savage, D. F.; Afonso, B.; Chen, A. H.; Silver, P. A. (2010). "Spatially Ordered Dynamics of the Bacterial Carbon Fixation Machinery". Science. 327 (5970): 1258–1261. doi:10.1126/science.1186090. ISSN 0036-8075.
- ↑ Cai, Fei; Dou, Zhicheng; Bernstein, Susan; Leverenz, Ryan; Williams, Eric; Heinhorst, Sabine; Shively, Jessup; Cannon, Gordon; Kerfeld, Cheryl (2015). "Advances in Understanding Carboxysome Assembly in Prochlorococcus and Synechococcus Implicate CsoS2 as a Critical Component". Life. 5 (2): 1141–1171. doi:10.3390/life5021141. ISSN 2075-1729.
- ↑ Iancu, Cristina V.; Morris, Dylan M.; Dou, Zhicheng; Heinhorst, Sabine; Cannon, Gordon C.; Jensen, Grant J. (2010). "Organization, Structure, and Assembly of α-Carboxysomes Determined by Electron Cryotomography of Intact Cells". Journal of Molecular Biology. 396 (1): 105–117. doi:10.1016/j.jmb.2009.11.019. ISSN 0022-2836.
- ↑ Nicole A. Leal, Gregory D. Havemann & Thomas A. Bobik (November 2003). "PduP is a coenzyme-a-acylating propionaldehyde dehydrogenase associated with the polyhedral bodies involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2". Archives of microbiology. 180 (5): 353–361. doi:10.1007/s00203-003-0601-0. PMID 14504694.
- ↑ Takamasa Tobimatsu, Masahiro Kawata & Tetsuo Toraya (March 2005). "The N-terminal regions of beta and gamma subunits lower the solubility of adenosylcobalamin-dependent diol dehydratase". Bioscience, Biotechnology, and Biochemistry. 69 (3): 455–462. doi:10.1271/bbb.69.455. PMID 15784971.
- ↑ Liu Y, Leal NA, Sampson EM, Johnson CL, Havemann GD, Bobik TA (March 2007). "PduL is an evolutionarily distinct phosphotransacylase involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar typhimurium LT2". Journal of Bacteriology. 189 (5): 1589–1596. doi:10.1128/JB.01151-06. PMID 17158662.
- ↑ Shibata, N.; Tamagaki, H.; Hieda, N.; Akita, K.; Komori, H.; Shomura, Y.; Terawaki, S.-i.; Mori, K.; Yasuoka, N.; Higuchi, Y.; Toraya, T. (2010). "Crystal Structures of Ethanolamine Ammonia-lyase Complexed with Coenzyme B12 Analogs and Substrates". Journal of Biological Chemistry. 285 (34): 26484–26493. doi:10.1074/jbc.M110.125112. ISSN 0021-9258.
- ↑ Aussignargues, Clément; Paasch, Bradley C.; Gonzalez-Esquer, Raul; Erbilgin, Onur; Kerfeld, Cheryl A. (2015). "Bacterial Microcompartment Assembly: The Key Role of Encapsulation Peptides". Communicative & Integrative Biology. 8: 00–00. doi:10.1080/19420889.2015.1039755. ISSN 1942-0889.
- 1 2 Fan, C.; Cheng, S.; Liu, Y.; Escobar, C. M.; Crowley, C. S.; Jefferson, R. E.; Yeates, T. O.; Bobik, T. A. (2010). "Short N-terminal sequences package proteins into bacterial microcompartments". Proceedings of the National Academy of Sciences. 107 (16): 7509–7514. doi:10.1073/pnas.0913199107. ISSN 0027-8424. PMC 2867708
 . PMID 20308536.
. PMID 20308536. - ↑ Fan, C.; Bobik, T. A. (2011). "The N-Terminal Region of the Medium Subunit (PduD) Packages Adenosylcobalamin-Dependent Diol Dehydratase (PduCDE) into the Pdu Microcompartment". Journal of Bacteriology. 193 (20): 5623–5628. doi:10.1128/JB.05661-11. ISSN 0021-9193.
- ↑ Choudhary, Swati; Quin, Maureen B.; Sanders, Mark A.; Johnson, Ethan T.; Schmidt-Dannert, Claudia (2012). "Engineered Protein Nano-Compartments for Targeted Enzyme Localization". PLoS ONE. 7 (3): e33342. doi:10.1371/journal.pone.0033342. ISSN 1932-6203.
- 1 2 Lassila, Jonathan K.; Bernstein, Susan L.; Kinney, James N.; Axen, Seth D.; Kerfeld, Cheryl A. (2014). "Assembly of Robust Bacterial Microcompartment Shells Using Building Blocks from an Organelle of Unknown Function". Journal of Molecular Biology. 426 (11): 2217–2228. doi:10.1016/j.jmb.2014.02.025. ISSN 0022-2836.
- 1 2 T. A. Bobik, M. Ailion & J. R. Roth (April 1992). "A single regulatory gene integrates control of vitamin B12 synthesis and propanediol degradation". Journal of Bacteriology. 174 (7): 2253–2266. PMID 1312999.
- ↑ M. Ailion, T. A. Bobik & J. R. Roth (November 1993). "Two global regulatory systems (Crp and Arc) control the cobalamin/propanediol regulon of Salmonella typhimurium". Journal of Bacteriology. 175 (22): 7200–7208. PMID 8226666.
- ↑ D. E. Sheppard & J. R. Roth (March 1994). "A rationale for autoinduction of a transcriptional activator: ethanolamine ammonia-lyase (EutBC) and the operon activator (EutR) compete for adenosyl-cobalamin in Salmonella typhimurium". Journal of Bacteriology. 176 (5): 1287–1296. PMID 8113167.
- ↑ Joseph B, Przybilla K, Stühler C, Schauer K, Slaghuis J, Fuchs TM, Goebel W (January 2006). "Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening". Journal of Bacteriology. 188 (2): 556–568. doi:10.1128/JB.188.2.556-568.2006. PMID 16385046.
- ↑ Jochen Klumpp & Thilo M. Fuchs (April 2007). "Identification of novel genes in genomic islands that contribute to Salmonella typhimurium replication in macrophages". Microbiology. 153 (Pt 4): 1207–1220. doi:10.1099/mic.0.2006/004747-0. PMID 17379730.
- ↑ Maadani A, Fox KA, Mylonakis E, Garsin DA (May 2007). "Enterococcus faecalis mutations affecting virulence in the Caenorhabditis elegans model host". Infection and immunity. 75 (5): 2634–2637. doi:10.1128/IAI.01372-06. PMID 17307944.
- ↑ Harvey, P. C.; Watson, M.; Hulme, S.; Jones, M. A.; Lovell, M.; Berchieri, A.; Young, J.; Bumstead, N.; Barrow, P. (2011). "Salmonella enterica Serovar Typhimurium Colonizing the Lumen of the Chicken Intestine Grows Slowly and Upregulates a Unique Set of Virulence and Metabolism Genes". Infection and Immunity. 79 (10): 4105–4121. doi:10.1128/IAI.01390-10. ISSN 0019-9567.
- ↑ Kendall, M. M.; Gruber, C. C.; Parker, C. T.; Sperandio, V. (2012). "Ethanolamine Controls Expression of Genes Encoding Components Involved in Interkingdom Signaling and Virulence in Enterohemorrhagic Escherichia coli O157:H7". mBio. 3 (3): e00050–12–e00050–12. doi:10.1128/mBio.00050-12. ISSN 2150-7511.
- ↑ Lin, Myat T.; Occhialini, Alessandro; Andralojc, P. John; Devonshire, Jean; Hines, Kevin M.; Parry, Martin A. J.; Hanson, Maureen R. (2014). "β-Carboxysomal proteins assemble into highly organized structures inNicotianachloroplasts". The Plant Journal. 79 (1): 1–12. doi:10.1111/tpj.12536. ISSN 0960-7412.
- ↑ Lin, Myat T.; Occhialini, Alessandro; Andralojc, P. John; Parry, Martin A. J.; Hanson, Maureen R. (2014). "A faster Rubisco with potential to increase photosynthesis in crops". Nature. 513 (7519): 547–550. doi:10.1038/nature13776. ISSN 0028-0836. PMID 25231869.
- ↑ Lawrence, Andrew D.; Frank, Stefanie; Newnham, Sarah; Lee, Matthew J.; Brown, Ian R.; Xue, Wei-Feng; Rowe, Michelle L.; Mulvihill, Daniel P.; Prentice, Michael B.; Howard, Mark J.; Warren, Martin J. (2014). "Solution Structure of a Bacterial Microcompartment Targeting Peptide and Its Application in the Construction of an Ethanol Bioreactor". ACS Synthetic Biology. 3 (7): 454–465. doi:10.1021/sb4001118. ISSN 2161-5063.
- ↑ Cai, Fei; Sutter, Markus; Bernstein, Susan L.; Kinney, James N.; Kerfeld, Cheryl A. (2015). "Engineering Bacterial Microcompartment Shells: Chimeric Shell Proteins and Chimeric Carboxysome Shells". ACS Synthetic Biology. 4 (4): 444–453. doi:10.1021/sb500226j. ISSN 2161-5063.
External links
- Mysterious Bacterial Microcompartments Revealed By Biochemists
- Not so simple after all. A renaissance of research into prokaryotic evolution and cell structure