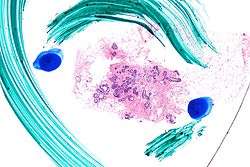
Atypical ductal hyperplasia
| Atypical ductal hyperplasia | |
|---|---|
|
Very low magnification micrograph of atypical ductal hyperplasia (ADH). The piece with ADH was circled by the pathologist with a marker, as it is so small, and sent for an additional opinion. H&E stain. | |
| Classification and external resources |
Atypical ductal hyperplasia, abbreviated ADH, is the term used for a benign lesion of the breast that indicates an increased risk of breast cancer.[1]
The name of the entity is descriptive of the lesion; ADH is characterized by cellular proliferation (hyperplasia) within one or two breast ducts and (histomorphologic) architectural abnormalities, i.e. the cells are arranged in an abnormal or atypical way.
In the context of a core (needle) biopsy, ADH is considered an indication for a breast lumpectomy, also known as a surgical (excisional) biopsy, to exclude the presence of breast cancer.[2]
Symptoms
ADH, generally, is asymptomatic. It usually comes to medical attention on a screening mammogram, as a non-specific suspicious abnormality that requires a biopsy.
Diagnosis
It is diagnosed based on tissue, e.g. a biopsy.[3] Histomorphologically, it has architectural changes seen in low-grade ductal carcinoma in situ (DCIS), e.g. cribriform architecture, and like low-grade DCIS has minimal nuclear atypia and no necrosis.
Pathology
ADH, cytologically, architecturally and on a molecular basis, is identical to a low-grade ductal carcinoma in situ (DCIS);[4] however, it has a limited extent, i.e. is present in a very small amount (< 2 mm).
Relation to low-grade ductal carcinoma in situ
While the histopathologic features and molecular features of ADH are that of (low-grade) DCIS, its clinical behaviour, unlike low-grade DCIS, is substantially better; thus, the more aggressive treatment for DCIS is not justified. In oncology in general, it is observed that tumour size is often strongly predictive of the clinical behaviour and, thus, a number of cancers (e.g. adenocarcinoma of the lung, papillary renal cell carcinoma) are defined, in part, on the basis of a minimum size.
Treatment
ADH, if found on a surgical (excisional) biopsy of a mammographic abnormality, does not require any further treatment, only mammographic follow-up.
If ADH is found on a core (needle) biopsy (a procedure which generally does not excise a suspicious mammographic abnormality), a surgical biopsy, i.e. a breast lumpectomy, to completely excise the abnormality and exclude breast cancer is the typical recommendation.
Prognosis
Cancer risk for ADH on a core biopsy
The rate at which breast cancer (ductal carcinoma in situ or invasive mammary carcinoma (all breast cancer except DCIS and LCIS)) is found at the time of a surgical (excisional) biopsy, following the diagnosis of ADH on a core (needle) biopsy varies considerably from hospital-to-hospital (range 4-54%).[5] In two large studies, the conversion of an ADH on core biopsy to breast cancer on surgical excision, known as "up-grading", is approximately 30%.[5][6]
Cancer risk based on follow-up
The relative risk of breast cancer based on a median follow-up of 8 years, in a case control study of US registered nurses, is 3.7.[7]
See also
Additional images
-

Low mag.
-
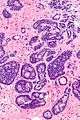
High mag.
References
- ↑ "Understanding Breast Changes - National Cancer Institute". Archived from the original on May 27, 2010.
- ↑ Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP (May 1995). "Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy". AJR Am J Roentgenol. 164 (5): 1111–3. doi:10.2214/ajr.164.5.7717215. PMID 7717215.
- ↑ Eby PR, Ochsner JE, DeMartini WB, Allison KH, Peacock S, Lehman CD (January 2009). "Frequency and upgrade rates of atypical ductal hyperplasia diagnosed at stereotactic vacuum-assisted breast biopsy: 9-versus 11-gauge". AJR Am J Roentgenol. 192 (1): 229–34. doi:10.2214/AJR.08.1342. PMID 19098204.
- ↑ Ghofrani, M.; Tapia, B.; Tavassoli, FA. (Dec 2006). "Discrepancies in the diagnosis of intraductal proliferative lesions of the breast and its management implications: results of a multinational survey". Virchows Arch. 449 (6): 609–16. doi:10.1007/s00428-006-0245-y. PMC 1888715
 . PMID 17058097.
. PMID 17058097. - 1 2 Deshaies, I.; Provencher, L.; Jacob, S.; Côté, G.; Robert, J.; Desbiens, C.; Poirier, B.; Hogue, JC.; Vachon, E. (Feb 2011). "Factors associated with upgrading to malignancy at surgery of atypical ductal hyperplasia diagnosed on core biopsy". Breast. 20 (1): 50–5. doi:10.1016/j.breast.2010.06.004. PMID 20619647.
- ↑ Margenthaler, JA.; Duke, D.; Monsees, BS.; Barton, PT.; Clark, C.; Dietz, JR. (Oct 2006). "Correlation between core biopsy and excisional biopsy in breast high-risk lesions". Am J Surg. 192 (4): 534–7. doi:10.1016/j.amjsurg.2006.06.003. PMID 16978969.
- ↑ London, SJ.; Connolly, JL.; Schnitt, SJ.; Colditz, GA. (Feb 1992). "A prospective study of benign breast disease and the risk of breast cancer". JAMA. 267 (7): 941–4. doi:10.1001/jama.1992.03480070057030. PMID 1734106.