Atrial fibrillation
| Atrial fibrillation | |
|---|---|
|
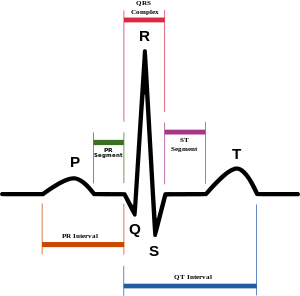
Diagram of normal sinus rhythm as seen on ECG. In atrial fibrillation the P waves, which represent depolarization of the top of the heart, are absent. | |
| Classification and external resources | |
| Specialty | Cardiology |
| ICD-10 | I48 |
| ICD-9-CM | 427.31 |
| DiseasesDB | 1065 |
| MedlinePlus | 000184 |
| eMedicine | med/184 emerg/46 |
| Patient UK | Atrial fibrillation |
| MeSH | D001281 |
Atrial fibrillation (AF or A-fib) is an abnormal heart rhythm characterized by rapid and irregular beating.[1] Often it starts as brief periods of abnormal beating which become longer and possibly constant over time.[2] Most episodes have no symptoms.[3] Occasionally there may be heart palpitations, fainting, lightheadedness, shortness of breath, or chest pain.[4] The disease is associated with an increased risk of heart failure, dementia, and stroke.[3] It is a type of supraventricular tachycardia.[5]
High blood pressure and valvular heart disease are the most common alterable risk factors for AF.[6][7] Other heart-related risk factors include heart failure, coronary artery disease, cardiomyopathy, and congenital heart disease.[6] In the developing world valvular heart disease often occurs as a result of rheumatic fever.[8] Lung-related risk factors include COPD, obesity, and sleep apnea.[3] Other factors include excess alcohol intake, diabetes mellitus, and thyrotoxicosis.[3][8] However, half of cases are not associated with one of these risks.[3] A diagnosis is made by feeling the pulse and may be confirmed using an electrocardiogram (ECG).[9] A typical ECG in AF shows no P waves and an irregular ventricular rate.[9]
AF is often treated with medications to slow the heart rate to a near normal range (known as rate control) or to convert the rhythm to normal sinus rhythm (known as rhythm control).[6] Electrical cardioversion can also be used to convert AF to a normal sinus rhythm and is often used emergently if the person is unstable.[10] Ablation may prevent recurrence in some people.[11] Depending on the risk of stroke either aspirin or anti-clotting medications such as warfarin or a novel oral anticoagulant may be recommended.[3] While these medications reduce this risk, they increase rates of major bleeding.[12]
Atrial fibrillation is the most common serious abnormal heart rhythm.[3] In Europe and North America, as of 2014, it affects about 2% to 3% of the population.[2] This is an increase from 0.4 to 1% of the population around 2005.[13] In the developing world about 0.6% of males and 0.4% of females are affected. The percentage of people with AF increases with age with 0.14% under 50 years old, 4% between 60 and 70 years old, and 14% over 80 years old being affected.[2] A-fib and atrial flutter resulted in 112,000 deaths in 2013, up from 29,000 in 1990.[14] The first known report of an irregular pulse was by Jean-Baptiste de Sénac in 1749. This was first documented by ECG in 1909 by Thomas Lewis.[3]
Signs and symptoms

AF is usually accompanied by symptoms related to a rapid heart rate. Rapid and irregular heart rates may be perceived as palpitations or exercise intolerance and occasionally may produce anginal chest pain (if the high heart rate causes ischemia). Other possible symptoms include congestive symptoms such as shortness of breath or swelling. The arrhythmia is sometimes only identified with the onset of a stroke or a transient ischemic attack (TIA). It is not uncommon for a patient to first become aware of AF from a routine physical examination or ECG, as it often does not cause symptoms.[13]
Since most cases of AF are secondary to other medical problems, the presence of chest pain or angina, signs and symptoms of hyperthyroidism (an overactive thyroid gland) such as weight loss and diarrhea, and symptoms suggestive of lung disease can indicate an underlying cause. A history of stroke or TIA, as well as high blood pressure, diabetes, heart failure, or rheumatic fever may indicate whether someone with AF is at a higher risk of complications.[13] The risk of a blood clot forming in the left atrium, breaking off, and then traveling in the bloodstream can be assessed using the CHADS2 score or CHA2DS2-VASc score.
Rapid heart rate
Presentation is similar to other forms of rapid heart rate and may be asymptomatic.[15] Palpitations and chest discomfort are common complaints.[15] The rapid uncoordinated heart rate may result in reduced cardiac output, with the heart being unable to provide adequate blood flow and therefore oxygen delivery to the rest of the body. Common symptoms of uncontrolled atrial fibrillation may include shortness of breath,[15] shortness of breath when lying flat, dizziness, and sudden onset of shortness of breath during the night. This may progress to swelling of the lower extremities, a manifestation of congestive heart failure. Due to inadequate cardiac output, individuals with AF may also complain of light-headedness,[15] may feel like they are about to faint, or may actually lose consciousness.
AF can cause respiratory distress due to congestion in the lungs. By definition, the heart rate will be greater than 100 beats per minute. Blood pressure may be variable, and often difficult to measure as the beat-by-beat variability causes problems for most digital (oscillometric) non-invasive blood pressure monitors. For this reason, when determining heart rate in AF, direct cardiac auscultation is recommended. Low blood pressure is most concerning and a sign that immediate treatment is required. Many of the symptoms associated with uncontrolled atrial fibrillation are a manifestation of congestive heart failure due to the reduced cardiac output. Respiratory rate will be increased in the presence of respiratory distress. Pulse oximetry may confirm the presence of hypoxia related to any precipitating factors such as pneumonia. Examination of the jugular veins may reveal elevated pressure (jugular venous distention). Lung exam may reveal crackles, which are suggestive of pulmonary edema. Heart exam will reveal a rapid irregular rhythm.
Causes
AF is linked to several forms of cardiovascular disease, but may occur in otherwise normal hearts. Cardiovascular factors known to be associated with the development of AF include high blood pressure, coronary artery disease, mitral stenosis (e.g., due to rheumatic heart disease or mitral valve prolapse), mitral regurgitation, left atrial enlargement, hypertrophic cardiomyopathy (HCM), pericarditis, congenital heart disease, and previous heart surgery. Additionally, lung diseases (such as pneumonia, lung cancer, pulmonary embolism, and sarcoidosis) are thought to play a role in certain people. Disorders of breathing during sleep such as obstructive sleep apnea (OSA) are also associated with AF.[16] Obesity is a risk factor for AF.[17] Hyperthyroidism and subclinical hyperthyroidism are associated with AF development.[18] Caffeine consumption does not appear to be associated with AF,[19] but excessive alcohol consumption ("binge drinking" or "holiday heart syndrome") is linked to AF.[20]
Genetics
A family history of AF may increase the risk of AF. A study of more than 2,200 people found an increased risk factor for AF of 1.85 for those that had at least one parent with AF.[21][22][23] Various genetic mutations may be responsible.[24][25]
Four types of genetic disorder are associated with atrial fibrillation:[26]
- Familial AF as a monogenic disease
- Familial AF presenting in the setting of another inherited cardiac disease (hypertrophic cardiomyopathy, dilated cardiomyopathy, familial amyloidosis)
- Inherited arrhythmic syndromes (congenital long QT syndrome, short QT syndrome, Brugada syndrome)
- Non-familial AF associated with genetic backgrounds (polymorphism in the ACE gene) that may predispose to atrial fibrillation
Pathophysiology
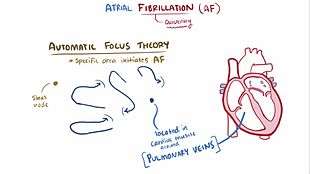
In AF, the normal regular electrical impulses generated by the sinoatrial node in the right atrium of the heart are overwhelmed by disorganized electrical impulses usually originating in the roots of the pulmonary veins. This leads to irregular conduction of ventricular impulses that generate the heartbeat.
Pathology
The primary pathologic change seen in atrial fibrillation is the progressive fibrosis of the atria. This fibrosis is due primarily to atrial dilation; however, genetic causes and inflammation may be factors in some individuals. Dilation of the atria can be due to almost any structural abnormality of the heart that can cause a rise in the pressure within the heart. This includes valvular heart disease (such as mitral stenosis, mitral regurgitation, and tricuspid regurgitation), hypertension, and congestive heart failure. Any inflammatory state that affects the heart can cause fibrosis of the atria. This is typically due to sarcoidosis but may also be due to autoimmune disorders that create autoantibodies against myosin heavy chains. Mutation of the lamin AC gene is also associated with fibrosis of the atria that can lead to atrial fibrillation.
Once dilation of the atria has occurred, this begins a chain of events that leads to the activation of the renin aldosterone angiotensin system (RAAS) and subsequent increase in matrix metalloproteinases and disintegrin, which leads to atrial remodeling and fibrosis, with loss of atrial muscle mass. This process is not immediate, and experimental studies have revealed patchy atrial fibrosis may precede the occurrence of atrial fibrillation and may progress with prolonged durations of atrial fibrillation.
Fibrosis is not limited to the muscle mass of the atria and may occur in the sinus node (SA node) and atrioventricular node (AV node), correlating with sick sinus syndrome. Prolonged episodes of atrial fibrillation have been shown to correlate with prolongation of the sinus node recovery time,[13] suggesting that dysfunction of the SA node is progressive with prolonged episodes of atrial fibrillation.
Electrophysiology
| Conduction | ||
Sinus rhythm  |
Atrial fibrillation 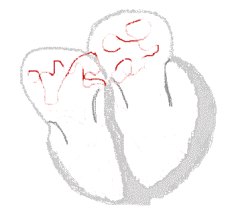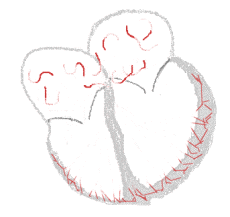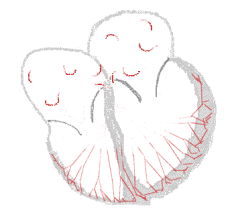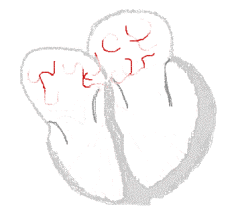 | |
The normal electrical conduction system of the heart allows the impulse that is generated by the sinoatrial node (SA node) of the heart to be propagated to and stimulate the myocardium (muscular layer of the heart). When the myocardium is stimulated, it contracts. It is the ordered stimulation of the myocardium that allows efficient contraction of the heart, thereby allowing blood to be pumped to the body.
There are multiple theories about the etiology of atrial fibrillation. An important theory is that, in atrial fibrillation, the regular impulses produced by the sinus node for a normal heartbeat are overwhelmed by rapid electrical discharges produced in the atria and adjacent parts of the pulmonary veins. Sources of these disturbances are either automatic foci, often localized at one of the pulmonary veins, or a small number of localized sources in the form of either reentrant electrical spiral waves (rotors) or repetitive focal beats; these localized sources may be found in the left atrium near the pulmonary veins or in a variety of other locations through both the left or right atrium.
Because recovery of the atria from excitation is heterogeneous, the electrical waves generated by the AF sources undergo repetitively, spatially distributed breakup and fragmentation in a process known as "fibrillatory conduction". Another theory is the multiple wavelet theory.[27]
AF can be distinguished from atrial flutter (AFL), which appears as an organized electrical circuit usually in the right atrium. AFL produces characteristic saw-toothed F-waves of constant amplitude and frequency on an ECG whereas AF does not. In AFL, the discharges circulate rapidly at a rate of 300 beats per minute (bpm) around the atrium. In AF, there is no regularity of this kind, except at the sources where the local activation rate can exceed 500 bpm.
Although the electrical impulses of AF occur at a high rate, most of them do not result in a heart beat. A heart beat results when an electrical impulse from the atria passes through the atrioventricular (AV) node to the ventricles and causes them to contract. During AF, if all of the impulses from the atria passed through the AV node, there would be severe ventricular tachycardia, resulting in a severe reduction of cardiac output. This dangerous situation is prevented by the AV node since its limited conduction velocity reduces the rate at which impulses reach the ventricles during AF.[28]
Diagnosis

The evaluation of atrial fibrillation involves a determination of the cause of the arrhythmia, and classification of the arrhythmia. Diagnostic investigation of AF typically includes a complete history and physical examination, ECG, transthoracic echocardiogram, complete blood count, and serum thyroid stimulating hormone level.[15] Depending upon given resources, afflicted individuals may benefit from an in-depth evaluation that may include correlation of the heart rate response to exercise, exercise stress testing, chest X-ray, transesophageal echocardiography, and other studies.
If a patient presents with a sudden onset of severe symptoms, other forms of abnormal heart rhythm with high heart rate must be ruled out, as some may be immediately life-threatening, such as ventricular tachycardia. While most patients will be placed on continuous cardiorespiratory monitoring, an ECG is essential for diagnosis. Provoking causes should be sought out. A common cause of any tachycardia is dehydration, as well as other forms of hypovolemia. Acute coronary syndrome should be ruled out. Intercurrent illness such as pneumonia may be present.
Screening
In general, screening for atrial fibrillation is not performed. Screening in those 65 years and older has been studied and been found to increase the number of cases of atrial fibrillation detected.[29]
Minimal evaluation
In general, the minimal evaluation of atrial fibrillation should be performed in all individuals with AF. The goal of this evaluation is to determine the general treatment regimen for the individual. If results of the general evaluation warrant it, further studies may then be performed.
History and physical examination
The history of the individual's atrial fibrillation episodes is probably the most important part of the evaluation. Distinctions should be made between those who are entirely asymptomatic when they are in AF (in which case the AF is found as an incidental finding on an ECG or physical examination) and those who have gross and obvious symptoms due to AF and can pinpoint whenever they go into AF or revert to sinus rhythm.
Routine bloodwork
While many cases of AF have no definite cause, it may be the result of various other problems. Hence, kidney function and electrolytes are routinely determined, as well as thyroid-stimulating hormone (commonly suppressed in hyperthyroidism and of relevance if amiodarone is administered for treatment) and a blood count.[13]
In acute-onset AF associated with chest pain, cardiac troponins or other markers of damage to the heart muscle may be ordered. Coagulation studies (INR/aPTT) are usually performed, as anticoagulant medication may be commenced.[13]
Electrocardiogram

Atrial fibrillation is diagnosed on an electrocardiogram (ECG), an investigation performed routinely whenever an irregular heart beat is suspected. Characteristic findings are the absence of P waves, with disorganized electrical activity in their place, and irregular R-R intervals due to irregular conduction of impulses to the ventricles.[13] At very fast heart rates atrial fibrillation may look more regular, which may make it more difficult to separate from SVT or ventricular tachycardia.[30]
QRS complexes should be narrow, signifying that they are initiated by normal conduction of atrial electrical activity through the intraventricular conduction system. Wide QRS complexes are worrisome for ventricular tachycardia, although, in cases where there is a disease of the conduction system, wide complexes may be present in A-Fib with rapid ventricular response.
If paroxysmal AF is suspected but an ECG during an office visit shows only a regular rhythm, AF episodes may be detected and documented with the use of ambulatory Holter monitoring (e.g., for a day). If the episodes are too infrequent to be detected by Holter monitoring with reasonable probability, then the patient can be monitored for longer periods (e.g., a month) with an ambulatory event monitor.[13]
Echocardiography
In general, a non-invasive transthoracic echocardiogram (TTE) is performed in newly diagnosed AF, as well as if there is a major change in the patient's clinical state. This ultrasound-based scan of the heart may help identify valvular heart disease (which may greatly increase the risk of stroke), left and right atrial size (which indicates likelihood that AF may become permanent), left ventricular size and function, peak right ventricular pressure (pulmonary hypertension), presence of left atrial thrombus (low sensitivity), presence of left ventricular hypertrophy and pericardial disease.[13]
Significant enlargement of both the left and right atria is associated with long-standing atrial fibrillation and, if noted at the initial presentation of atrial fibrillation, suggests that the atrial fibrillation is likely to be of a longer duration than the individual's symptoms.
Extended evaluation
In general, an extended evaluation is not necessary for most individuals with atrial fibrillation and is performed only if abnormalities are noted in the limited evaluation, if a reversible cause of the atrial fibrillation is suggested, or if further evaluation may change the treatment course.
Chest X-ray
In general, a chest X-ray is performed only if a pulmonary cause of atrial fibrillation is suggested, or if other cardiac conditions are suspected (in particular congestive heart failure.) This may reveal an underlying problem in the lungs or the blood vessels in the chest.[13] In particular, if an underlying pneumonia is suggested, then treatment of the pneumonia may cause the atrial fibrillation to terminate on its own.
Transesophageal echocardiogram
A regular echocardiogram (transthoracic echo/TTE) has a low sensitivity for identifying blood clots in the heart. If this is suspected (e.g., when planning urgent electrical cardioversion) a transesophageal echocardiogram/TEE (or TOE where British spelling is used) is preferred.[13]
The TEE has much better visualization of the left atrial appendage than transthoracic echocardiography. This structure, located in the left atrium, is the place where a blood clot forms in more than 90% of cases in non-valvular (or non-rheumatic) atrial fibrillation.[31][32] TEE has a high sensitivity for locating thrombi in this area[33] and can also detect sluggish bloodflow in this area that is suggestive of blood clot formation.[34]
If no blood clot is seen on TEE, the incidence of stroke immediately after cardioversion is performed is very low.
Ambulatory Holter monitoring
A Holter monitor is a wearable ambulatory heart monitor that continuously monitors the heart rate and heart rhythm for a short duration, typically 24 hours. In individuals with symptoms of significant shortness of breath with exertion or palpitations on a regular basis, a Holter monitor may be of benefit to determine whether rapid heart rates (or unusually slow heart rates) during atrial fibrillation are the cause of the symptoms.
Exercise stress testing
Some individuals with atrial fibrillation do well with normal activity but develop shortness of breath with exertion. It may be unclear whether the shortness of breath is due to a blunted heart rate response to exertion caused by excessive atrioventricular node-blocking agents, a very rapid heart rate during exertion, or other underlying conditions such as chronic lung disease or coronary ischemia. An exercise stress test will evaluate the individual's heart rate response to exertion and determine if the AV node blocking agents are contributing to the symptoms.
Classification
| AF category | Defining characteristics |
|---|---|
| First detected | only one diagnosed episode |
| Paroxysmal | recurrent episodes that stop on their own in less than 7 days |
| Persistent | recurrent episodes that last more than 7 days |
| Permanent | an ongoing long-term episode |
The American College of Cardiology (ACC), American Heart Association (AHA), and the European Society of Cardiology (ESC) recommend in their guidelines the following classification system based on simplicity and clinical relevance.[13]
All people with AF are initially in the category called first detected AF. These patients may or may not have had previous undetected episodes. If a first detected episode stops on its own in less than 7 days and then another episode begins, later on, the category changes to paroxysmal AF. Although patients in this category have episodes lasting up to 7 days, in most cases of paroxysmal AF the episodes will stop in less than 24 hours. If the episode lasts for more than 7 days, it is unlikely to stop on its own,[35] and is then known as persistent AF. In this case, cardioversion can be used to stop the episode. If cardioversion is unsuccessful or not attempted and the episode continues for a long time (e.g., a year or more), the patient's AF is then known as permanent.
Episodes that last less than 30 seconds are not considered in this classification system. Also, this system does not apply to cases where the AF is a secondary condition that occurs in the setting of a primary condition that may be the cause of the AF.
About half of people with AF have permanent AF, while a quarter have paroxysmal AF, and a quarter have persistent AF.[2]
In addition to the above four AF categories, which are mainly defined by episode timing and termination, the ACC/AHA/ESC guidelines describe additional AF categories in terms of other characteristics of the patient.[13]
- Lone atrial fibrillation (LAF) – absence of clinical or echocardiographic findings of other cardiovascular disease (including hypertension), related pulmonary disease, or cardiac abnormalities such as enlargement of the left atrium, and age under 60 years
- Nonvalvular AF – absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair
- Secondary AF – occurs in the setting of a primary condition that may be the cause of the AF, such as acute myocardial infarction, cardiac surgery, pericarditis, myocarditis, hyperthyroidism, pulmonary embolism, pneumonia, or other acute pulmonary disease
Management
The main goals of treatment are to prevent circulatory instability and stroke. Rate or rhythm control are used to achieve the former, whereas anticoagulation is used to decrease the risk of the latter.[36] If cardiovascularly unstable due to uncontrolled tachycardia, immediate cardioversion is indicated.[13] An exercise program may be useful.
Anticoagulants
Anticoagulation can be used to reduce the risk of stroke from AF. Anticoagulation is recommended in most people other than those at low risk of stroke[37] or those at high risk of bleeding.
The risk of stroke from non-valvular AF can be estimated using the CHA2DS2-VASc score. A 2014 AHA/ACC/HRS guideline said that for nonvalvular AF, anticoagulation is recommended if there is a score of 2 or more, not using anticoagulation may be considered if there is a score of 1, and not using anticoagulation is reasonable if there is a score of 0.[38]
Anticoagulation can be achieved through a number of means including warfarin,[39] heparin, dabigatran, rivaroxaban[40] edoxaban,[41] and apixaban.[42] Aspirin is less effective in reducing the risk of stroke and may not be safer with respect to major bleeding (including intracranial bleeding) than well-managed warfarin or a non-vitamin K oral anticoagulant (NOAC).[43] A number of issues should be considered, including the cost of NOACs, risk of stroke, risk of falls, compliance, and speed of desired onset of anticoagulation.[44]
For those with non-valvular atrial fibrillation, the NOACs (rivaroxaban, dabigatran, apixaban) are neither superior nor worse than warfarin in preventing non-hemorrhagic stroke and systemic embolic events.[45][46] They have a lower risk of intracranial bleeding compared to warfarin; however, dabigatran is associated with a higher risk of gastrointestinal bleeding.[45][46]
Rate versus rhythm control
There are two ways to approach atrial fibrillation using medications: rate control and rhythm control. Both methods have similar outcomes.[47] Rate control lowers the heart rate closer to normal, usually 60 to 100 bpm, without trying to convert to a regular rhythm. Rhythm control tries to restore a normal heart rhythm in a process called cardioversion and maintains the normal rhythm with medications. Studies suggest that rhythm control is more important in the acute setting AF, whereas rate control is more important in the chronic phase.
There is no difference in risk of stroke in people having converted to a normal rhythm with antiarrhythmic treatment compared to those with only rate control.[48] AF is associated with a reduced quality of life, and, while some studies indicate that rhythm control leads to a higher quality of life, some did not find a difference.[49]
A further study focused on rhythm control in people with AF with heart failure, based on the idea that AF increases mortality in this group. In this setting, rhythm control offered no advantage compared to rate control.[50]
In those with a fast ventricular response, intravenous magnesium significantly increases the chances of successful rate and rhythm control in the urgent setting without major side-effects.[51] A person with poor vital signs, mental status changes, preexcitation, or chest pain often will go to immediate treatment with synchronized DC cardioversion.[13] Otherwise the decision of rate control versus rhythm control using drugs is made. This is based on a number of criteria that includes whether or not symptoms persist with rate control.
Rate control
Rate control to a target heart rate of 110 bpm is recommended in most people.[52] Lower heart rates may be recommended in those with left ventricular hypertrophy or reduced left ventricular function.[53] Rate control is achieved with medications that work by increasing the degree of block at the level of the AV node, decreasing the number of impulses that conduct into the ventricles. This can be done with:[13][54]
- Beta blockers (preferably the "cardioselective" beta blockers such as metoprolol, atenolol, bisoprolol, nebivolol)
- Non-dihydropyridine calcium channel blockers (e.g., diltiazem or verapamil)
- Cardiac glycosides (e.g., digoxin) – have less use, apart from in older people who are sedentary. They are not as effective as either beta blockers or calcium channel blockers.[6]
In those with chronic disease either beta blockers or calcium channel blockers are recommended.[52]
In addition to these agents, amiodarone has some AV node blocking effects (in particular when administered intravenously), and can be used in individuals when other agents are contraindicated or ineffective (particularly due to hypotension).
Cardioversion
Cardioversion is the attempt to switch an irregular heartbeat to a normal heartbeat using electrical or chemical means.[13]
- Electrical cardioversion involves the restoration of normal heart rhythm through the application of a DC electrical shock. Exact placement of the pads does not appear important.[55]
- Chemical cardioversion is performed with drugs, such as amiodarone, dronedarone,[56] procainamide, dofetilide, ibutilide, propafenone, or flecainide.
After successful cardioversion the heart may be in a stunned state, which means that there is a normal rhythm but restoration of normal atrial contraction has not yet occurred.[57]
Surgery
Ablation
In young people with little-to-no structural heart disease where rhythm control is desired and cannot be maintained by medication or cardioversion, then radiofrequency ablation or cryoablation may be attempted and is preferred over years of drug therapy.[13][58] Although radiofrequency ablation is becoming an accepted intervention in selected younger patients, there is currently a lack of evidence that ablation reduces all-cause mortality, stroke, or heart failure.[59] There are two ongoing clinical trials (CABANA [Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation] and EAST [Early Therapy of Atrial Fibrillation for Stroke Prevention Trial]) that should provide new information for assessing whether AF catheter ablation is superior to more standard therapy.[60]
The Maze procedure, first performed in 1987, is an effective invasive surgical treatment that is designed to create electrical blocks or barriers in the atria of the heart, forcing electrical impulses that stimulate the heartbeat to travel down to the ventricles. The idea is to force abnormal electrical signals to move along one, uniform path to the lower chambers of the heart (ventricles), thus restoring the normal heart rhythm.[61]
AF often occurs after cardiac surgery and is usually self-limiting. It is strongly associated with age, preoperative hypertension, and the number of vessels grafted. Measures should be taken to control hypertension preoperatively to reduce the risk of AF. Also, people with a higher risk of AF, e.g., people with pre-operative hypertension, more than 3 vessels grafted, or greater than 70 years of age, should be considered for prophylactic treatment. Postoperative pericardial effusion is also suspected to be the cause of atrial fibrillation. Prophylaxis may include prophylactic postoperative rate and rhythm management. Some authors perform posterior pericardiotomy to reduce the incidence of postoperative AF.[62] When AF occurs, management should primarily be rate and rhythm control. However, cardioversion may be employed if the person is hemodynamically unstable, highly symptomatic, or persists for 6 weeks after discharge. In persistent cases, anticoagulation should be used.
Left atrial appendage occlusion
There is tentative evidence that left atrial appendage occlusion therapy may reduce the risk of stroke in people with non-valvular AF as much as warfarin.[63]
Prognosis
Atrial fibrillation increases the risk of heart failure by 11 per 1000, kidney problems by 6 per 1000, death by 4 per 1000, stroke by 3 per 1000, and coronary heart disease by 1 per 1000.[64] Women have a worse outcome overall than men.[65]
Blood clots
Prediction of embolism
Determining the risk of an embolism causing a stroke is important for guiding the use of anticoagulants. The most accurate clinical prediction rules are:[66]
Both the CHADS2 and the CHA2DS2-VASc score predict future stroke risk in people with a-fib with CHA2DS2-VASc being more accurate. Some that had a CHADS2 score of 0 had a CHA2DS2-VASc score of 3, with a 3.2% annual risk of stroke. Thus a CHA2DS2-VASc score of 0 is considered very low risk.[67]
Mechanism of thrombus formation
In atrial fibrillation, the lack of an organized atrial contraction can result in some stagnant blood in the left atrium (LA) or left atrial appendage (LAA). This lack of movement of blood can lead to thrombus formation (blood clotting). If the clot becomes mobile and is carried away by the blood circulation, it is called an embolus. An embolus proceeds through smaller and smaller arteries until it plugs one of them and prevents blood from flowing through the artery. This process results in end organ damage due to loss of nutrients, oxygen, and removal of cellular waste products. Emboli in the brain may result in an ischemic stroke or a transient ischemic attack (TIA).
More than 90% of cases of thrombi associated with non-valvular atrial fibrillation evolve in the left atrial appendage.[31] However, the LAA lies in close relation to the free wall of the left ventricle and thus the LAA's emptying and filling, which determines its degree of blood stagnation, may be helped by the motion of the wall of the left ventricle, if there is good ventricular function.[68]
If the LA is enlarged, there is an increased risk of thrombi that originate in the LA. Moderate to severe, non-rheumatic, mitral regurgitation (MR) reduces this risk of stroke.[69] This risk reduction may be due to a beneficial swirling effect of the MR blood flow into the LA.[70]
Mitral valve
Atrial fibrillation and a corresponding enlargement of the left atrium may cause an increase in size of the mitral valve annulus.[71]
With a sinus rhythm, the mitral annulus undergoes dynamic changes during the cardiac cycle. For example, at the end of diastole the annular area is smaller than at the end of systole. A possible reason for this dynamic size difference is that the coordinated contraction of the left atrium acts like a sphincter about the mitral annulus and reduces its size. This may be important for mitral valve competence so that it does not leak when the left ventricle pumps blood. However, when the left atrium fibrillates, this sphincter action is not possible and may contribute to, or result in, mitral regurgitation in some cases.[71]
Epidemiology
Atrial fibrillation is the most common arrhythmia.[13] In Europe and North America as of 2014 it affects about 2% to 3% of the population.[2] This is an increase from 0.4 to 1% of the population around 2005.[13] In the developing world rates are about 0.6% for males and 0.4% for females.[2]
It also accounts for one-third of hospital admissions for cardiac rhythm disturbances,[13] and the rate of admissions for AF has risen in recent years.[72] Strokes from AF account for 6–24% of all ischemic strokes.[73] After a transient ischemic attack or stroke about 11% are found to have a new diagnosis of atrial fibrillation.[74] Between 3 and 11% of those with AF have structurally normal hearts.[75] Approximately 2.2 million individuals in the United States and 4.5 million in the European Union have AF.[13]
The number of new cases each year of atrial fibrillation increases with age. In individuals over the age of 80 it affects about 8%.[13] In developed countries, the number of patients with atrial fibrillation is likely to increase during the next 50 years, owing to the growing proportion of elderly individuals.[76]
History
Because the diagnosis of atrial fibrillation requires measurement of the electrical activity of the heart, atrial fibrillation was not truly described until 1874, when Edmé Félix Alfred Vulpian observed the irregular atrial electrical behavior that he termed "fremissement fibrillaire" in dog hearts.[77] In the mid-eighteenth century, Jean-Baptiste de Sénac made note of dilated, irritated atria in people with mitral stenosis.[78] The irregular pulse associated with AF was first recorded in 1876 by Carl Wilhelm Hermann Nothnagel and termed "delirium cordis", stating that "[I]n this form of arrhythmia the heartbeats follow each other in complete irregularity. At the same time, the height and tension of the individual pulse waves are continuously changing".[79] Correlation of delirium cordis with the loss of atrial contraction as reflected in the loss of a waves in the jugular venous pulse was made by Sir James MacKenzie in 1904.[80] Willem Einthoven published the first ECG showing AF in 1906.[81] The connection between the anatomic and electrical manifestations of AF and the irregular pulse of delirium cordis was made in 1909 by Carl Julius Rothberger, Heinrich Winterberg, and Sir Thomas Lewis.[82][83][84]
See also
References
- ↑ "Heart Disease Other Related Conditions". cdc.gov. September 3, 2014. Retrieved 19 February 2015.
- 1 2 3 4 5 6 Zoni-Berisso, M; Lercari, F; Carazza, T; Domenicucci, S (2014). "Epidemiology of atrial fibrillation: European perspective.". Clinical epidemiology. 6: 213–20. doi:10.2147/CLEP.S47385. PMID 24966695.
- 1 2 3 4 5 6 7 8 Munger, TM; Wu, LQ; Shen, WK (January 2014). "Atrial fibrillation.". Journal of biomedical research. 28 (1): 1–17. doi:10.7555/JBR.28.20130191. PMID 24474959.
- ↑ Gray, David (2010). Chamberlain's Symptoms and Signs in Clinical Medicine: An Introduction to Medical Diagnosis (13th ed.). London: Hodder Arnold. pp. 70–1. ISBN 9780340974254.
- ↑ Urman, edited by Linda S. Aglio, Robert W. Lekowski, Richard D. (2015). Essential clinical anesthesia review : keywords, questions and answers for the boards. p. 480. ISBN 9781107681309.
- 1 2 3 4 Anumonwo, JM; Kalifa, J (November 2014). "Risk Factors and Genetics of Atrial Fibrillation.". Cardiology clinics. 32 (4): 485–494. doi:10.1016/j.ccl.2014.07.007. PMID 25443231.
- ↑ Nguyen, TN; Hilmer, SN; Cumming, RG (10 September 2013). "Review of epidemiology and management of atrial fibrillation in developing countries.". International Journal of Cardiology. 167 (6): 2412–20. doi:10.1016/j.ijcard.2013.01.184. PMID 23453870.
- 1 2 Mischke, K; Knackstedt, C; Marx, N; Vollmann, D (April 2013). "Insights into atrial fibrillation.". Minerva medica. 104 (2): 119–30. PMID 23514988.
- 1 2 Ferguson C, Inglis SC, Newton PJ, Middleton S, Macdonald PS, Davidson PM (2013). "Atrial fibrillation: stroke prevention in focus". ACC. 00 (2): 92–98. doi:10.1016/j.aucc.2013.08.002. PMID 24054541.
- ↑ Oishi, ML; Xing, S (February 2013). "Atrial fibrillation: management strategies in the emergency department.". Emergency medicine practice. 15 (2): 1–26; quiz 27. PMID 23369365.
- ↑ Amerena, JV; Walters, TE; Mirzaee, S; Kalman, JM (4 November 2013). "Update on the management of atrial fibrillation.". The Medical journal of Australia. 199 (9): 592–97. doi:10.5694/mja13.10191. PMID 24182224.
- ↑ Steinberg, BA; Piccini, JP (14 April 2014). "Anticoagulation in atrial fibrillation.". BMJ (Clinical research ed.). 348: g2116. doi:10.1136/bmj.g2116. PMID 24733535.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Fuster, Valentin (2006). "ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society". Circulation. 114 (7): e257–354. doi:10.1161/CIRCULATIONAHA.106.177292. PMID 16908781.
- ↑ GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013.". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604
 . PMID 25530442.
. PMID 25530442. - 1 2 3 4 5 Gutierrez C, Blanchard DG (January 2011). "Atrial Fibrillation: Diagnosis and Treatment". Am Fam Physician (Review). 83 (1): 61–68. PMID 21888129.
- ↑ Abed HS, Wittert GA (November 2013). "Obesity and atrial fibrillation". Obesity Reviews. 14 (11): 929–38. doi:10.1111/obr.12056. PMID 23879190.
- ↑ Magnani JW, Hylek EM, Apovian CM (July 23, 2013). "Obesity begets atrial fibrillation: a contemporary summary" (PDF). Circulation. American Heart Association. 128 (4): 401–05. doi:10.1161/CIRCULATIONAHA.113.001840. PMID 23877062. Retrieved July 19, 2015.
- ↑ Palmeiro C, Davila MI, Bhat M, Frishman WH, Weiss IA (December 2013). "Subclinical hyperthyroidism and cardiovascular risk: recommendations for treatment". Cardiology in review. 21 (6): 300–08. doi:10.1097/CRD.0b013e318294f6f1. PMID 23563523.
- ↑ Cheng, M; Hu, Z; Lu, X; Huang, J; Gu, D (April 2014). "Caffeine intake and atrial fibrillation incidence: dose response meta-analysis of prospective cohort studies.". The Canadian journal of cardiology. 30 (4): 448–54. doi:10.1016/j.cjca.2013.12.026. PMID 24680173.
- ↑ Tonelo D, Providência R, Gonçalves L (August 2013). "Holiday heart syndrome revisited after 34 years". Arquivos brasileiros de cardiologia. 101 (2): 183–89. doi:10.5935/abc.20130153. PMC 3998158
 . PMID 24030078.
. PMID 24030078. - ↑ Fox CS, Parise H, D'Agostino RB, et al. (2004). "Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring". JAMA. 291 (23): 2851–55. doi:10.1001/jama.291.23.2851. PMID 15199036.
- ↑ Roberts JD, Gollob MH (2014). "A contemporary review on the genetic basis of atrial fibrillation". Methodist Debakey Cardiovasc J. 10: 18–24. doi:10.14797/mdcj-10-1-18. PMC 4051329
 . PMID 24932358.
. PMID 24932358. - ↑ Howlett PJ, Hatch FS, Alexeenko V, Jabr RI, Leatham EW, Fry CH (2015). "Diagnosing Paroxysmal Atrial Fibrillation: Are Biomarkers the Solution to This Elusive Arrhythmia?". Biomed Res Int. 2015: 910267. doi:10.1155/2015/910267. PMC 4502272
 . PMID 26229966.
. PMID 26229966. - ↑ Saffitz JE (2006). "Connexins, conduction, and atrial fibrillation". N. Engl. J. Med. 354 (25): 2712–14. doi:10.1056/NEJMe068088. PMID 16790707.
- ↑ "OMIM Online Mendelian Inheritance of Man". The National Center for Biotechnology Information. Retrieved 2010-08-24.
- ↑ Shimizu W (2013). "Atrial fibrillation and genetic abnormalities". Nihon Rinsho. 71 (1): 161–66.
- ↑ Fuster, Valentin (October 2001). "ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology". Circulation. 104 (17): 2118–50. PMID 11673357.
- ↑ Klabunde, Richard (2005). Cardiovascular Physiology Concepts. Lippincott Williams & Wilkins. pp. 25, 28. ISBN 978-0-7817-5030-1.
- ↑ Moran, PS; Flattery, MJ; Teljeur, C; Ryan, M; Smith, SM (Apr 30, 2013). "Effectiveness of systematic screening for the detection of atrial fibrillation.". The Cochrane database of systematic reviews. 4: CD009586. doi:10.1002/14651858.CD009586.pub2. PMID 23633374.
- ↑ Issa ZF, Miller JM, Zipes DP (2009). Clinical arrhythmology and electrophysiology : a companion to Braunwald's heart disease. Philadelphia: Saunders. p. 221. ISBN 978-1-4160-5998-1.
- 1 2 Blackshear JL, Odell JA (February 1996). "Appendage ligation to reduce stroke in cardiac surgical patients with atrial fibrillation". Ann. Thorac. Surg. 61 (2): 755–59. doi:10.1016/0003-4975(95)00887-X. PMID 8572814.
- ↑ Ramlawi, B; Abu Saleh, WK; Edgerton, J (2015). "The Left Atrial Appendage: Target for Stroke Reduction in Atrial Fibrillation.". Methodist DeBakey cardiovascular journal. 11 (2): 100–03. doi:10.14797/mdcj-11-2-100. PMID 26306127.
- ↑ Acar J, Cormier B, Grimberg D, et al. (1991). "Diagnosis of left atrial thrombi in mitral stenosis – usefulness of ultrasound techniques compared with other methods". European Heart Journal. 12 (Supplement B) (Jul): 70–06. doi:10.1093/eurheartj/12.suppl_B.70. PMID 1936030. Retrieved 2009-10-20.
- ↑ Fatkin D, Kelly RP, Feneley MP (1994). "Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo". Journal of the American College of Cardiology. 23 (4): 961–69. doi:10.1016/0735-1097(94)90644-0. PMID 8106703. Retrieved 2009-10-21.
- ↑ Levy S (2000). "Classification system of atrial fibrillation". Current Opinion in Cardiology. 15 (1): 54–57. doi:10.1097/00001573-200001000-00007. PMID 10666661.
- ↑ Prystowsky, Eric N; Padanilam, Benzy J; Fogel, MD, Richard I (July 21, 2015). "Treatment of Atrial Fibrillation". JAMA. 314 (3): 278–88. doi:10.1001/jama.2015.7505. PMID 26197188.
- ↑ Lip, GY; Lane, DA (19 May 2015). "Stroke prevention in atrial fibrillation: a systematic review.". JAMA. 313 (19): 1950–62. doi:10.1001/jama.2015.4369. PMID 25988464.
- ↑ January et al. 2014, pp. e211–e212
- ↑ Ciervo CA, Granger CB, Schaller FA (September 2012). "Stroke prevention in patients with atrial fibrillation: disease burden and unmet medical needs". J Am Osteopath Assoc (Review). 112 (9 (Suppl 2)): eS2–8. PMID 23014814. Archived from the original on 2016-03-05.
- ↑ "FDA approves Xarelto to prevent stroke in people with common type of abnormal heart rhythm". FDA. Retrieved Nov 4, 2011.
- ↑ "FDA approves anti-clotting drug Savaysa". FDA. Retrieved 23 June 2016/ref>. Check date values in:
|access-date=(help) - ↑ "FDA approves Eliquis to reduce the risk of stroke, blood clots in patients with non-valvular atrial fibrillation". FDA. Retrieved 2012-12-30.
- ↑ Lip, GY (26 July 2011). "The role of aspirin for stroke prevention in atrial fibrillation.". Nature reviews. Cardiology. 8 (10): 602–06. doi:10.1038/nrcardio.2011.112. PMID 21788962.
- ↑ Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ (Sep 2004). "Antithrombotic Therapy in Atrial Fibrillation : The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy". Chest. 126 (3_suppl): 429S–56S. doi:10.1378/chest.126.3_suppl.429S. PMID 15383480. Retrieved 2012-10-02.
- 1 2 Sharma, M; Cornelius, VR; Patel, JP; Davies, JG; Molokhia, M (20 May 2015). "Efficacy and Harms of Direct Oral Anticoagulants in the Elderly for Stroke Prevention in Atrial Fibrillation and Secondary Prevention of Venous Thromboembolism: Systematic Review and Meta-Analysis.". Circulation. 132: 194–204. doi:10.1161/CIRCULATIONAHA.114.013267. PMID 25995317.
- 1 2 Gómez-Outes, A; Terleira-Fernández, AI; Calvo-Rojas, G; Suárez-Gea, ML; Vargas-Castrillón, E (2013). "Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups.". Thrombosis. 2013: 640723. doi:10.1155/2013/640723. PMC 3885278
 . PMID 24455237.
. PMID 24455237. - ↑ Al-Khatib, Sana M. (June 3, 2014). "Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review.". Annals of Internal Medicine. 160 (11): 760–73. doi:10.7326/M13-1467. PMID 24887617.
- ↑ Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD (2002). "A comparison of rate control and rhythm control in patients with atrial fibrillation - The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators". N Engl J Med. 347 (23): 1825–33. doi:10.1056/NEJMoa021328. PMID 12466506.
- ↑ Thrall G, Lane D, Carroll D, Lip GY (2006). "Quality of life in patients with atrial fibrillation: a systematic review". Am. J. Med. 119 (5): 448.e1–19. doi:10.1016/j.amjmed.2005.10.057. PMID 16651058.
- ↑ Roy, Denis (June 2008). "Rhythm control versus rate control for atrial fibrillation and heart failure". N Engl J Med. 358 (25): 2667–77. doi:10.1056/NEJMoa0708789. PMID 18565859.
- ↑ Onalan O, Crystal E, Daoulah A, Lau C, Crystal A, Lashevsky I (2007). "Meta-analysis of magnesium therapy for the acute management of rapid atrial fibrillation". Am. J. Cardiol. 99 (12): 1726–32. doi:10.1016/j.amjcard.2007.01.057. PMID 17560883.
- 1 2 Anderson, JL; Halperin, JL; Albert, NM; Bozkurt, B; Brindis, RG; Curtis, LH; DeMets, D; Guyton, RA; Hochman, JS; Kovacs, RJ; Ohman, EM; Pressler, SJ; Sellke, FW; Shen, WK; Wann, LS; Curtis, AB; Ellenbogen, KA; Estes NA, 3rd; Ezekowitz, MD; Jackman, WM; January, CT; Lowe, JE; Page, RL; Slotwiner, DJ; Stevenson, WG; Tracy, CM; Fuster, V; Rydén, LE; Cannom, DS; Crijns, HJ; Curtis, AB; Ellenbogen, KA; Le Heuzey, JY; Kay, GN; Olsson, SB; Prystowsky, EN; Tamargo, JL; Wann, S (7 May 2013). "Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.". Journal of the American College of Cardiology. 61 (18): 1935–44. doi:10.1016/j.jacc.2013.02.001. PMID 23558044.
- ↑ Badheka, AO; Shah, N; Grover, PM; Patel, NJ; Chothani, A; Mehta, K; Singh, V; Deshmukh, A; Savani, GT; Rathod, A; Panaich, SS; Patel, N; Arora, S; Bhalara, V; Coffey, JO; Mitrani, RD; Halperin, JL; Viles-Gonzalez, JF (1 April 2014). "Outcomes in atrial fibrillation patients with and without left ventricular hypertrophy when treated with a lenient rate-control or rhythm-control strategy.". The American journal of cardiology. 113 (7): 1159–65. doi:10.1016/j.amjcard.2013.12.021. PMID 24507168.
- ↑ "Atrial fibrillation: national clinical guideline for management in primary and secondary care" (PDF). National Collaborating Centre for Chronic Conditions. London: Royal College of Physicians. 2006.
- ↑ Kirkland, S; Stiell, I; AlShawabkeh, T; Campbell, S; Dickinson, G; Rowe, BH (July 2014). "The efficacy of pad placement for electrical cardioversion of atrial fibrillation/flutter: a systematic review.". Academic Emergency Medicine. 21 (7): 717–26. doi:10.1111/acem.12407. PMID 25117151.
- ↑ Bramah N. Singh (2007). "Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter". N. Engl. J. Med. 357 (10): 987–99. doi:10.1056/NEJMoa054686. PMID 17804843.
- ↑ Watson T, Shantsila E, Lip GY (10 Jan 2009). "Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited". Lancet. 373 (9658): 155–66. doi:10.1016/S0140-6736(09)60040-4. PMID 19135613.
- ↑ Leong-Sit P, Zado E, Callans DJ, et al. "Efficacy and risk of atrial fibrillation ablation before 45 years of age". Circ Arrhythm Electrophysiol. 2010. 3:452–57.
- ↑ Agency for Healthcare Research and Quality. Research Protocol: Treatment of Atrial Fibrillation. 2012; December 2012.
- ↑ January, CT; Wann, LS; Alpert, JS; Calkins, H; Cigarroa, JE; Cleveland, JC Jr; Conti, JB; Ellinor, PT; Ezekowitz, MD; Field, ME; Murray, KT; Sacco, RL; Stevenson, WG; Tchou, PJ; Tracy, CM; Yancy, CW (March 28, 2014). "2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society". Circulation. American Heart Association, Inc. 130: e199–e267. doi:10.1161/CIR.0000000000000041. Executive summary: PMID 24682348
- ↑ Northwestern Surgery for Atrial Fibrillation. Atrial Fibrillation Surgery
- ↑ Kaleda VI, McCormack DJ, Shipolini AR (April 2012). "Does posterior pericardiotomy reduce the incidence of atrial fibrillation after coronary artery bypass grafting surgery?". Interact. Cardiovasc. Thorac. Surg. 14 (4): 384–89. doi:10.1093/icvts/ivr099. PMC 3309809
 . PMID 22235005.
. PMID 22235005. - ↑ Zhou, X; Zhang, W; Lv, W; Zhou, Q; Li, Y; Zhang, L; Lu, Y; Zhang, J; Xing, Q; Wang, H; Tang, B (15 January 2016). "Left atrial appendage occlusion in atrial fibrillation for stroke prevention: A systemic review.". International Journal of Cardiology. 203: 55–59. doi:10.1016/j.ijcard.2015.10.011. PMID 26492310.
- ↑ Odutayo, Ayodele; Wong, Christopher X; Hsiao, Allan J; Hopewell, Sally; Altman, Douglas G; Emdin, Connor A (6 September 2016). "Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis". BMJ: i4482. doi:10.1136/bmj.i4482.
- ↑ Emdin, CA; Wong, CX; Hsiao, AJ; Altman, DG; Peters, SA; Woodward, M; Odutayo, AA (19 January 2016). "Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies.". BMJ (Clinical research ed.). 532: h7013. PMID 26786546.
- ↑ Lopes RD, Crowley MJ, Shah BR, et al . Stroke Prevention in Atrial Fibrillation. Comparative Effectiveness Review No. 123. AHRQ Publication No. 13-EHC113-EF. Rockville, MD: Agency for Healthcare Research and Quality; August 2013. www.effectivehealthcare.ahrq.gov/ reports/final.cfm.
- ↑ Olesen, JB; Torp-Pedersen, C; Hansen, ML; Lip, GY (2012). "The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study.". Thromb Haemost. 107 (6): 1172–79. doi:10.1160/th12-03-0175. PMID 22473219.
- ↑ Al-Saady, N. M.; O. A. Abel; A. J. Camm (1999). "Left atrial appendage: structure, function, and role in thromboembolism". Heart. 82 (5): 547–55. doi:10.1136/hrt.82.5.547. PMC 1760793
 . PMID 10525506.
. PMID 10525506. - ↑ Nakagami H, Yamamoto K, Ikeda U, Mitsuhashi T, Goto T, Shimada K (1998). "Mitral regurgitation reduces the risk of stroke in patients with nonrheumatic atrial fibrillation". American Heart Journal. 136 (3): 528–32. doi:10.1016/S0002-8703(98)70231-5. PMID 9736148. Retrieved 2010-02-23.
- ↑ Cheng, TO (November 1999). "Reduced risk for thromboembolism in atrial fibrillation and mitral regurgitation". American Heart Journal. 138 (5 Pt 1): 998–99. doi:10.1016/S0002-8703(99)70045-1. PMID 10539836.
- 1 2 Pai, RG; Varadarajan, P; Tanimoto, M (January 2003). "Effect of Atrial Fibrillation on the Dynamics of Mitral Annular Area". The Journal of Heart Valve Disease. 12 (1): 31–37. PMID 12578332. Retrieved 2009-12-20.
- ↑ Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB (2003). "Rising rates of hospital admissions for atrial fibrillation". Epidemiology. 14 (6): 666–72. doi:10.1097/01.ede.0000091649.26364.c0. PMID 14569181.
- ↑ Narumiya T, Sakamaki T, Sato Y, Kanmatsuse K (January 2003). "Relationship between left atrial appendage function and left atrial thrombus in patients with nonvalvular chronic atrial fibrillation and atrial flutter". Circulation Journal. 67 (1): 68–72. doi:10.1253/circj.67.68. PMID 12520155.
- ↑ Kishore, A; Vail, A; Majid, A; Dawson, J; Lees, KR; Tyrrell, PJ; Smith, CJ (Feb 2014). "Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis.". Stroke; a journal of cerebral circulation. 45 (2): 520–26. doi:10.1161/STROKEAHA.113.003433. PMID 24385275.
- ↑ Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, Weyman AE (1990). "Atrial enlargement as a consequence of atrial fibrillation A prospective echocardiographic study". Circulation. 82 (3): 792–97. doi:10.1161/01.CIR.82.3.792. PMID 2144217. Archived from the original on 1 December 2009. Retrieved 2009-12-02.
- ↑ Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE (2001). "Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study". JAMA. 285 (18): 2370–75. doi:10.1001/jama.285.18.2370. PMID 11343485.
- ↑ Vulpian A (1874). "Note sur les effets de la faradisation directe des ventricules du coeur chez le chien". Archives de Physiologie Normale et Pathologique. 6: 975.
- ↑ McMichael J (1982). "History of atrial fibrillation 1628–1819 Harvey – de Senac – Laënnec". Br Heart J. 48 (3): 193–97. doi:10.1136/hrt.48.3.193. PMC 481228
 . PMID 7049202.
. PMID 7049202. - ↑ Nothnagel H (1876). "Ueber arythmische Herzthatigkeit". Deutsches Archiv für Klinische Medizin. 17: 190–220.
- ↑ MacKenzie J (1904). "Observations on the Inception of the Rhythm of the Heart by the Ventricle: As the cause of Continuous Irregularity of the Heart". Br Med J. 1 (2253): 529–36. doi:10.1136/bmj.1.2253.529. PMC 2353402
 . PMID 20761393.
. PMID 20761393. - ↑ Einthoven W (1906). "Le telecardiogramme". Archives Internationales de Physiologie. 4: 132–64.
- ↑ Rothberger CJ, Winterberg H (1909). "Vorhofflimmern und Arhythmia perpetua". Wiener Klinische Wochenschrift. 22: 839–44.
- ↑ Lewis T (1909). "Auricular fibrillation: a common clinical condition". Br Med J. 2 (2552): 1528. doi:10.1136/bmj.2.2552.1528.
- ↑ Flegel KM (1995). "From delirium cordis to atrial fibrillation: historical development of a disease concept". Ann. Intern. Med. 122 (11): 867–73. doi:10.7326/0003-4819-122-11-199506010-00010. PMID 7741373.
External links
| Wikimedia Commons has media related to Atrial fibrillation. |
- Atrial fibrillation at DMOZ
- "Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.". Circulation. 127 (18): 1916–26. 7 May 2013. doi:10.1161/CIR.0b013e318290826d. PMID 23545139.
- CHADS2 Score for Atrial Fibrillation Stroke Risk
- CHA2DS2-VASc Score for Atrial Fibrillation Stroke Risk