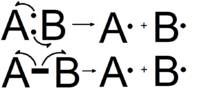Arrow pushing
Arrow pushing or electron pushing is a technique used to describe the progression of organic chemistry reaction mechanisms.[1] It was first developed by Sir Robert Robinson.[2] In using arrow pushing, "curved arrows" or "curly arrows" are superimposed over the structural formulae of reactants in a chemical equation to show the reaction mechanism. The arrows illustrate the movement of electrons as bonds between atoms are broken and formed. Arrow pushing is also used to describe how positive and negative charges are distributed around organic molecules through resonance. It is important to always remember, however, that arrow pushing is a formalism and electrons (or rather, electron density) do not move around so neatly and discretely in reality.
Recently, arrow pushing has been extended to inorganic chemistry, especially to the chemistry of s- and p-block elements. It has been shown to work well for hypervalent compounds.[3]
Notation
Organic chemists use two types of arrows within molecular structures to describe electron movements. Single electrons' trajectories are designated with single barbed arrows, whereas double-barbed arrows show movement of electron pairs.

When a bond is broken, electrons leave where the bond was and this is represented by a curved arrow pointing away from the bond and ending the arrow pointing towards the next unoccupied molecular orbital . Similarly, organic chemists represent the formation of a bond by a curved arrow pointing between two species.[4]
For clarity, when pushing arrows, it is best to draw the arrows starting from a lone pair of electrons or filled bonds (sigma, pi) and ending in an unfilled molecular orbital, allowing the reader to know exactly which electrons are moving and where they are ending.
Breaking of bonds
A covalent bond joining atoms in an organic molecule consists of a group of two electrons. Such a group is referred to as an electron pair. Reactions in organic chemistry proceed through the sequential breaking and formation of such bonds. Organic chemists recognize two processes for the breaking of a chemical bond. These processes are known as homolytic cleavage and heterolytic cleavage.[5]gg
Homolytic bond cleavage
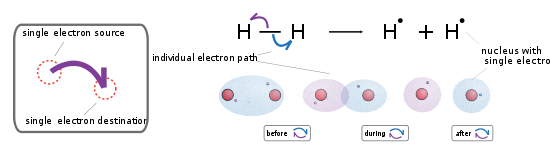
Homolytic bond cleavage is a process where the electron pair comprising a bond is split, causing the bond to break. This is denoted by two single barbed curved arrows pointing away from the bond. The consequence of this process is the retention of a single unpaired electron on each of the atoms that were formerly joined by a bond. These single electron species are known as free radicals.
For example, Ultraviolet light causes the chlorine-chlorine bond to break homolytically. This is the initiation stage of free radical halogenation.
Heterolytic bond cleavage
Heterolytic bond cleavage is a process where the electron pair that comprised a bond moves to one of the atoms that was formerly joined by a bond. The bond breaks, forming a negatively charged species (an anion) and a positively charged species (a cation). The anion is the species that retains the electrons from the bond while the cation is stripped of the electrons from the bond. The anion usually forms on the most electronegative atom, in this example atom A.
Heterolytic Reaction Mechanisms
All heterolytic organic chemistry reactions can be described by a sequence of fundamental mechanistic subtypes. The elementary mechanistic subtypes taught in introductory organic chemistry are SN1, SN2, E1, E2, addition and addition-elimination. Using arrow pushing, each of these mechanistic subtypes can be described.
SN1 reactions
An SN1 reaction occurs when a molecule separates into a positively charged component and a negatively charged component. This generally occurs in highly polar solvents through a process called solvolysis. The positively charged component then reacts with a nucleophile forming a new compound.
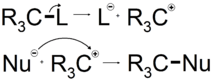
In the first stage of this reaction (solvolysis), the C-L bond breaks and both electrons from that bond join L (the leaving group) to form L− and R3C+ ions. This is represented by the curved arrow pointing away from the C-L bond and towards L. The nucleophile Nu−, being attracted to the R3C+, then donates a pair of electrons forming a new C-Nu bond.
Because an SN1 reaction proceeds with the Substitution of a leaving group with a Nucleophile, the SN designation is used. Because the initial solvolysis step in this reaction involves a single molecule dissociating from its leaving group, the initial stage of this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to SN1.
SN2 reactions
An SN2 reaction occurs when a nucleophile displaces a leaving group residing on a molecule. This displacement or substitution results in the formation of a new compound.
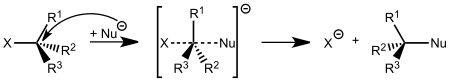
Because an SN2 reaction proceeds with the Substitution of a leaving group with a Nucleophile, the SN designation is used. Because this mechanism proceeds with the initial approach of two species, it is referred to as a bi-molecular reaction. The involvement of 2 species enhances the mechanistic designation to SN2.
E1 eliminations
An E1 elimination occurs when a proton adjacent to a positive charge leaves and generates a double bond.

Because initial formation of a cation is necessary for E1 reactions to occur, E1 reactions are often observed as side reactions to SN1 mechanisms.

E1 eliminations proceed with the Elimination of a leaving group leading to the E designation. Because this mechanism proceeds with the initial dissociation of a single starting material forming a carbocation, this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to E1.
E2 eliminations
An E2 elimination occurs when a proton adjacent to a leaving group is extracted by a base with simultaneous elimination of a leaving group and generation of a double bond.
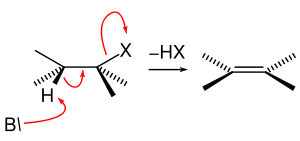
Similar to the relationship between E1 eliminations and SN1 mechanisms, E2 eliminations often occur in competition with SN2 reactions. This observation is most often noted when the base is also a nucleophile. In order to minimize this competition, non-nucleophilic bases are commonly used to effect E2 eliminations.
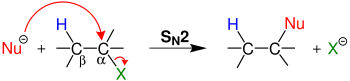
E2 eliminations proceed through initial extraction of a proton by a base or nucleophile leading to Elimination of a leaving group justifying the E designation. Because this mechanism proceeds through the interaction of two species (substrate and base/nucleophile), E2 reactions are recognized as bi-molecular. Thus, the involvement of 2 species in the initial phase of the reaction enhances the mechanistic designation to E2.
Addition reactions
Addition reactions occur when nucleophiles react with carbonyls. When a nucleophile adds to a simple aldehyde or ketone, the result is a 1,2-addition. When a nucleophile adds to a conjugated carbonyl system, the result is a 1,4-addition. The designations 1,2 and 1,4 are derived from numbering the atoms of the starting compound where the oxygen is labeled “1” and each atom adjacent to the oxygen are sequentially numbered out to the site of nucleophilic addition. A 1,2-addition occurs with nucleophilic addition to position 2 while a 1,4-addition occurs with nucleophilic addition to position 4.
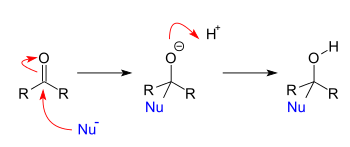
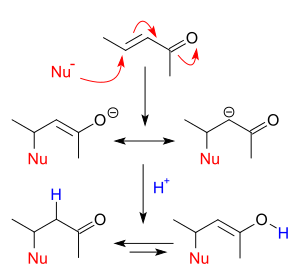
Addition-elimination reactions
Addition-elimination reactions are addition reactions immediately followed by elimination reactions. In general, these reactions take place when esters (or related functional groups) react with nucleophiles. In fact, the only requirement for an addition-elimination reaction to proceed is that the group being eliminated is a better leaving group than the incoming nucleophile.
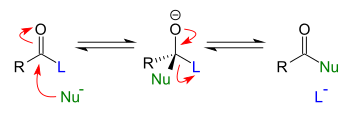
See also
Notes
- ↑ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. pp. 123–133. ISBN 978-0-19-850346-0.
- ↑ "Sir Robert Robinson's drawing of arrows".
- ↑ Abhik Ghosh, Steffen Berg, Arrow Pushing in Inorganic Chemistry: A Logical Approach to the Chemistry of the Main Group Elements, (John Wiley & Sons, 2014)
- ↑ "Notes on arrow pushing (curly arrows)" (PDF). Imperial College London. Retrieved 2009-04-27.
- ↑ "Free Radical Reactions -- One Electron Intermediates". Washington State University. Retrieved 2009-05-02.
References
- Daniel E. Levy, Arrow Pushing in Organic Chemistry: An Easy Approach to Understanding Reaction Mechanisms, (John Wiley & Sons, 2008)
- Daniel P. Weeks, Pushing Electrons: A Guide for Students of Organic Chemistry, (Brooks Cole, 1998)
- Abhik Ghosh, Steffen Berg, Arrow Pushing in Inorganic Chemistry: A Logical Approach to the Chemistry of the Main Group Elements, (John Wiley & Sons, 2014)
- Robert B. Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, (Springer, 2007)
External links
- MIT.edu, OpenCourseWare: Organic Chemistry I
- HaverFord.edu, Organic Chemistry Lectures, Videos and Text
- CEM.MSU.edu, Virtual Textbook of Organic Chemistry