Bioenergetic systems
Bioenergetic systems are metabolic processes that relate to the flow of energy in living organisms. Those processes convert energy into adenosine triphosphate (ATP), which is the form suitable for muscular activity. There are two main forms of synthesis of ATP: aerobic, which involves oxygen from the bloodstream, and anaerobic, which does not. Bioenergetics is the field of biology that studies bioenergetic systems.
Overview
The cellular respiration process that converts food energy into ATP (a form of energy) is largely dependent on oxygen availability. During exercise, the supply and demand of oxygen available to muscle cells is affected by duration and intensity and by the individual's cardiorespiratory fitness level. Three exercise energy systems can be selectively recruited, depending on the amount of oxygen available, as part of the cellular respiration process to generate the ATP for the muscles. They are ATP, the anaerobic system and the aerobic system.
Adenosine triphosphate
ATP is the usable form of chemical energy for muscular activity. It is stored in most cells, particularly in muscle cells. Other forms of chemical energy, such as those available from food, must be transformed into ATP before they can be utilized by the muscle cells.[1]
Coupled reactions
Since energy is released when ATP is broken down, energy is required to rebuild or resynthesize it. The building blocks of ATP synthesis are the by-products of its breakdown; adenosine diphosphate (ADP) and inorganic phosphate (Pi). The energy for ATP resynthesis comes from three different series of chemical reactions that take place within the body. Two of the three depend upon the type of food eaten, whereas the other depends upon a chemical compound called phosphocreatine. The energy released from any of these three series of reactions is coupled with the energy needs of the reaction that resynthesizes ATP. The separate reactions are functionally linked together in such a way that the energy released by the one is always used by the other.[1]:8–9
Three methods can synthesize ATP:
- ATP–CP system (phosphogen system) – This system is used for durations of up to 10 seconds. The ATP–CP system neither uses oxygen or produces lactic acid if oxygen is unavailable and is thus said to be alactic anaerobic. This is the primary system behind very short, powerful movements like a golf swing, a 100 m sprint or powerlifting.
- Anaerobic system – Predominates in supplying energy for exercises lasting less than two minutes. Also known as the glycolytic system. An example of an activity of the intensity and duration that this system works under would be a 400 m sprint.
- Aerobic system – This is the long-duration energy system. After five minutes of exercise, the O2 system is dominant. In a 1 km run, this system is already providing approximately half the energy; in a marathon run it provides 98% or more.[2]
Aerobic and anaerobic systems usually work concurrently. When describing activity, it is not a question of which energy system is working, but which predominates.[3]
Aerobic and anaerobic metabolism
The term metabolism refers to the various series of chemical reactions that take place within the body. Aerobic refers to the presence of oxygen, whereas anaerobic means with series of chemical reactions that does not require the presence of oxygen. The ATP-CP series and the lactic acid series are anaerobic, whereas the oxygen series is aerobic.[1]:9
ATP–CP: the phosphagen system
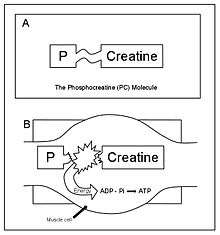
Creatine phosphate (CP), like ATP, is stored in muscle cells. When it is broken down, a large amount of energy is released. The energy released is coupled to the energy requirement necessary for the resynthesis of ATP.
The total muscular stores of both ATP and CP are small. Thus, the amount of energy obtainable through this system is limited. The phosphogen stored in the working muscles is typically exhausted in seconds of vigorous activity. However, the usefulness of the ATP-CP system lies in the rapid availability of energy rather than quantity. This is important with respect to the kinds of physical activities that humans are capable of performing.[1]:9–11
Anaerobic system
This system is known as anaerobic glycolysis. "Glycolysis" refers to the breakdown of sugar. In this system, the breakdown of sugar supplies the necessary energy from which ATP is manufactured. When sugar is metabolized anaerobically, it is only partially broken down and one of the byproducts is lactic acid. This process creates enough energy to couple with the energy requirements to resynthesize ATP.
When H+ ions accumulate in the muscles causing the blood pH level to reach very low levels, temporary muscle fatigue results. Another limitation of the lactic acid system that relates to its anaerobic quality is that only a few moles of ATP can be resynthesized from the breakdown of sugar as compared to the yield possible when oxygen is present. This system cannot be relied on for extended periods of time.
The lactic acid system, like the ATP-CP system, is important primarily because it provides a rapid supply of ATP energy. For example, exercises that are performed at maximum rates for between 1 and 3 minutes depend heavily upon the lactic acid system for ATP energy. In activities such as running 1500 meters or a mile, the lactic acid system is used predominately for the "kick" at the end of a race.[1]:11–12
Aerobic system
- The Krebs cycle
- Oxidative phosphorylation
- Glycolysis – The first stage is known as glycolysis, which produces 2 ATP molecules, 2 reduced molecules of nicotinamide adenine dinucleotide (NADH) and 2 pyruvate molecules that move on to the next stage – the Krebs cycle. Glycolysis takes place in the cytoplasm of normal body cells, or the sarcoplasm of muscle cells.
- The Krebs cycle – This is the second stage, and the products of this stage of the aerobic system are a net production of one ATP, one carbon dioxide molecule, three reduced NAD molecules, one reduced nicotinamide adenine dinucleotide FAD molecule (The molecules of NAD and FAD mentioned here are electron carriers, and if they are said to be reduced, this means that they have had one H+ ion added to them). The metabolites are for each turn of the Krebs cycle. The Krebs cycle turns twice for each molecule of glucose that passes through the aerobic system – as two pyruvate molecules enter the Krebs cycle. In order for the pyruvate molecules to enter the Krebs cycle they must be converted to acetyl coenzyme A. During this link reaction, for each molecule of pyruvate that gets converted to acetyl coenzyme A, a NAD is also reduced. This stage of the aerobic system takes place in the matrix of the cells' mitochondria.
- Oxidative phosphorylation – The last stage of the aerobic system produces the largest yield of ATP out of all the stages – a total of 34 ATP molecules. It is called oxidative phosphorylation because oxygen is the final acceptor of the electrons and hydrogen ions that leave this stage of aerobic respiration (hence oxidative) and ADP gets phosphorylated (an extra phosphate gets added) to form ATP (hence phosphorylation).
This stage of the aerobic system occurs on the cristae (infoldings on the membrane of the mitochondria). The NADH+ from glycolysis and the Krebs cycle, and the FADH+ from the Krebs cycle produce electron carriers at decreasing energy levels, in which energy is released to reform ATP. Each NADH+ that travels this electron transport chain provides enough energy for 3 molecules of ATP, and each molecule of FADH+ provides enough energy for 2 molecules of ATP. If you do your math this means that 10 total NADH+ molecules allow the rejuvenation of 30 ATP, and 2 FADH+ molecules allow for 4 ATP molecules to be rejuvenated (the total being 34 from oxidative phosphorylation, plus the 4 from the previous 2 stages meaning a total of 38 ATP being produced during the aerobic system). The NADH+ and FADH+ get oxidized to allow the NAD and FAD to return to be used in the aerobic system again, and electrons and hydrogen ions are accepted by oxygen to produce water, a harmless byproduct.
References
- 1 2 3 4 5 Edward L. Fox (1979). Sports physiology. Saunders College Publishing. ISBN 978-0-7216-3829-4.
- ↑ "James Madison University Strength and Conditioning Program". Archived from the original on 2008-04-20.
- ↑ Energy Proportion Graphs
Further reading
- Exercise Physiology for Health, Fitness and Performance. Sharon Plowman and Denise Smith. Lippincott Williams & Wilkins; Third edition (2010). ISBN 978-0-7817-7976-0.