Amphidromus
| Amphidromus | |
|---|---|
 | |
| Abapertural and apertural view of the shell of Amphidromus perversus | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Gastropoda |
| (unranked): | clade Heterobranchia clade Euthyneura clade Panpulmonata clade Eupulmonata clade Stylommatophora informal group Sigmurethra |
| Superfamily: | Helicoidea |
| Family: | Camaenidae |
| Subfamily: | Camaeninae |
| Genus: | Amphidromus Albers, 1850[1] |
| Diversity[2][3][4] | |
| 87 species | |
Amphidromus is a genus of air-breathing land snails, terrestrial pulmonate gastropod mollusks in the family Camaenidae.
Amphidromus is a genus of arboreal pulmonate land snails. The shells of Amphidromus are relatively large, from one to three inches high, and colorful.
Definition of Amphidromus
The genus may be characterized as follows:[5] It has brightly colored, elongate conic shells, dextral or sinistral with one to three inches high with 5 to 8 whorls which increase regularly in size. The aperture is sublunate or auriform, without teeth or folds, height two-fifths to one-third that of shell. The peristome is expanded and reflected, sometimes thickened. Parietal callus is weak to well-developed. The umbilicus is open or closed. Color pattern is monochrome to variegated. The jaw is thin and weak with low flat ribs. The radula is with cusped teeth arranged in V-shaped rows and modified in typical arboreal pattern. The pallial region is sigmurethrous with very long, narrow kidney. Genitalia are camaenid, with long seminal receptacle, and short penis with low insertion of retractor muscle. The habitat is arboreal.[5]
History
- 18th century
The shells of Amphidromus are relatively large and colorful and were among the first Indonesian land snails brought back to Europe; most specimens in collections were brought back by travelers or explorers during the 18th century.[5] Comparatively little material has been gathered by malacologists.[5] Several species and forms were described before 1800, generally with inadequate locality data.[5] At least two kinds — Amphidromus laevus (Müller, 1774) and the form Amphidromus perversus f. aureus Martyn, 1784 — still have not been reported from a precise locality.[5]
- 19th century
Many species and varieties were named during the first half of the nineteenth century, again usually with poor locality data. Not until Eduard von Martens' (1867)[6] publication was there an effort to deal with the entire complex. This paper showed an amazing grasp of variation and problems of geographic distribution, and many of von Martens' concepts are still utilized.[5] Hugh Fulton (1896)[7] organized 142 specific and varietal names into eighteen species groups with 64 species. When the monograph Manual of Conchology of Henry Augustus Pilsbry (1900)[8] appeared, the number of species had increased to 81, which were placed in nineteen groups. Pilsbry's study has remained the only illustrated monograph of the genus, and it is still indispensable for any serious study.[5]
- 20th century
Since 1900, the major taxonomic studies on Amphidromus have been faunistic (a study of the fauna of some territory or area) in scope. The papers of Paul Bartsch (1917, 1918, 1919)[9][10][11] on the Philippine species, Bernhard Rensch (1932)[12] on the Lesser Sunda Islands forms, and Tera van Benthem Jutting (1950, 1959)[13][14] on Javan and Sumatran populations are especially comprehensive. Potentially the most valuable[5] contribution is that of Curt Haniel (1921),[15] who discussed the variation of Amphidromus contrarius and Amphidromus reflexilabris on Timor. Variations in color and form were well illustrated in a series of color plates. Many of later conclusions by Frank Fortescue Laidlaw & Alan Solem (1961)[5] concerning the relationships of color forms described as species were taken not as much from new collections as from the extent of variation found by Haniel (1921) in his pioneer study. Adolf Michael Zilch (1953)[16] listed type specimens in the Senckenberg Museum and illustrated many previously unfigured species. The literature contains many scattered descriptions of new color forms and subspecies that appeared after 1900.[5] Of the 309 names in the nomenclatural list, 111 (35.9%) were published after Pilsbry (1900).[5] Frank Fortescue Laidlaw & Alan Solem (1961)[5] recognized 74 species by name and considered that material from the Banda Islands probably represents an undescribed species. There are eleven species recognized by them that were described after the appearance of Pilsbry's monograph.[5] Several species recognized by Pilsbry we have subordinated to subspecific or varietal status, and a few names have been transferred to incertae sedis, since they are based on hundred-year-old references that have not been substantiated by more recent collectors.[5] Study by Frank Fortescue Laidlaw & Alan Solem (1961)[5] forms a supplement to Pilsbry's monograph with his extensive plates.[5]
Distribution
Distribution of the genus Amphidromus include from eastern India in south-western Asia to northern Australia.[17]
Amphidromus is found from the Garo Hills and Khasi Hills of Meghalaya in northeastern India, throughout Burma, Malay Peninsula, Thailand, Laos, Cambodia, Vietnam, Indonesia as far east as the Sulawesi, Banda Islands, Timor and the Tenimber Islands (but not on Ceram, Buru, Halmahera, Batjan Island, the Obi Islands, the Aru and Kei Islands, or the Talaud Archipelago and some Celebesian satellite islands), in the southern Philippines, notably Mindanao and the Balabac, Palawan,[5] and in northern Australia.[17]
Fossil distribution
No pre-human fossil occurrences have been recorded.[5] Tera van Benthem Jutting (1932)[18] reported several specimens of Amphidromus filozonatus that had been eaten by natives from Sampoeng Cave, central Java, and later (van Benthem Jutting, 1937, pp. 92–94)[19] a single specimen of Amphidromus palaceus from the Trinil Beds of Java.[5] Neither record ante dates human occupancy, and thus yields no record on the early history of Amphidromus.[5]
Shell description
The shells of Amphidromus are relatively large, from one to three inches high, and colorful.[5] Amphidromus has an elongate conic or ovate conic helicoid shell of 5 to 8 whorls.[5] The shell may be thin and fragile or very heavy and solid with no known correlation of shell structure with distribution or habitats.[5]
In some species the shell coils invariably to the right, and in many others just as invariably to the left.[5] A significant number of species however are "amphidromine".[5] That is to say, both left- and right-handed coiling are found in the same population (they are polymorphic for direction of the shell coiling, but as there are only two possible types of shell coiling, they are known as dimorphic in coiling), sometimes in approximately equal numbers, other times with a distinct predominance of one phase.[5] In Amphidromus there is no information on the heredity of this character.[5]
Most species of other amphidromine species such as Partula and Achatinella have become extinct,[2] so the genus Amphidromus with its over 80 species is unique and useful for study of the evolution of asymmetry in animals.[2] This is also the reason for urge of this genus conservation.[2]
 Amphidromus floresianus from the subgenus Syndromus is normally only sinistral. |
 Amphidromine Amphidromus perversus can be dextral... |
 ...but Amphidromus perversus can also be sinistral. |
The whorls of Amphidromus are moderately convex and, with few exceptions, smooth or with only a faint sculpture of growth lines.[5] At least four separate times, however, a sculpture of moderately heavy oblique radial ribs has appeared (Amphidromus costifer Smith from Binh Dinh Province in Vietnam, Amphidromus begini Morlet from Cambodia, Amphidromus heccarii Tapparone-Canefri from Celebes, and the Amphidromus palaceus-Amphidromus winteri complex from Java and Sumatra).[5] Correlated with the ribbing is light, monochrome coloration, thin shell with large aperture, and flaring lip.[5] Many solid shells show a slight roughening of the surface, but this is quite different from the ribbed sculpture mentioned above.[5]
The aperture is generally large and varies from about two-fifths to one-third the height of the shell, often in the same population.[5] Usually the lip is at least somewhat expanded, and in forms such as Amphidromus reflexilahris Schepman and Amphidromus winteri (Pfeiffer) var. inauris Fulton, the lip can only be called flaring.[5] In Amphidromus perversus (Linnaeus) and most other thick-shelled species, the lip is internally thickened, forms a "roll" in its expansion, and has a very heavy parietal callus.[5] In thin-shelled species, the lip is usually a simple reflected edge.[5] The umbilical area may be partially open, nearly closed or sealed.[5] This sometimes provides a handy criterion for specific identification.[5] The angle of the parietal wall varies, but no precise information on this has been compiled.[5]
Generally the whorls increase rather regularly in size, but probably related species such as Amphidromus sinistralis (Reeve) and Amphidromus heccarii Tapparone-Canefri can have quite different degrees of whorl increment.[5] No attempt has been made to express these differences meristically, since most of the available material was inadequate for statistical treatment (but see Haniel, 1921).[5] Actual dimensions vary greatly both within and between species.[5] Minimum adult size is about 21 mm high, the observed maximum about 75 mm.[5] Only a few species, notably Amphidromus maculiferus, Amphidromus sinensis and Amphidromus entobaptus, seem to have a range in adult size of more than seven or eight millimeters.[5]
The major shell variation is found in the color pattern.[5] Most arboreal snails are brightly colored, the bulimulid genera Drymaeus and Liguus, cepolid Polymita, and the camaenid Papuina being obvious examples.[5] Polymita, Liguus and Amphidromus are noted for their color variations.[5]
The basic ground color of Amphidromus seems to be yellow and is usually (except for Amphidromus entobaptus) confined to the surface layers of the shell, since worn specimens appear to be nearly devoid of color.[5] Many species are whitish, and a few have dark ground colors.[5] The apical whorls are pale, purple, brown, or black; they sometimes vary within a population (Amphidromus quadrasi).[5] A few species, for example, Amphidromus schomburgki, have a deciduous green periostracum.[5] Continuous zonal patterns can take the form of whitish subsutural bands (Amphidromus similis), heavy subperipheral pigmentation (Amphidromus perversus var. infraviridis), subsutural color lines (Amphidromus columellaris), broad spiral color bands (Amphidromus metabletus, Amphidromus webbi), or narrow spiral bands (Amphidromus laevus).[5] Interrupted zonation can be the interruption of bands into spots (Amphidromus maculatus), highly irregular splitting of zones (Amphidromus perversus vars. sultanus and interruptus), formation of oblique radial streaks which can parallel (Amphidromus inversus) or cross (Amphidromus latestrigatus) the incremental growth lines, or almost every conceivable combination and variation of these factors.[5] Often the pattern will change radically from the apex to the body whorl (Amphidromus quadrasi vars.).[5] Aperture, parietal callus, columella, lip, and umbilical region are variously marked with pink, brown, purple, white, or black. Haniel (1921)[15] has several color plates which show effectively the extent of color variation within two species of the Syndromus type.[5] Amphidromus perversus and Amphidromus maculiferus of the Amphidromus subgenus are equally variable, while species such as Amphidromus inversus and Amphidromus similis are almost uniform in coloration.[5]
Most of the Amphidromus subgenus show resting stages by the deposition of a brown or black radial band called a varix.[5] This is apparently rare in the Syndromus subgenus, although Amphidromus laevus shows evidence of interruption of the spiral banding after a resting phase.[5]
Species recognition is based on combinations of minor structural variations in the shape, aperture, whorl contour, umbilical region, and color pattern.[5] Apparently, many species have a stable color pattern, while others seem to vary tremendously.[5] Adequate unselected field samples will enable a better understanding of the relative stability or variability of particular species in single localities.[5]
Anatomy
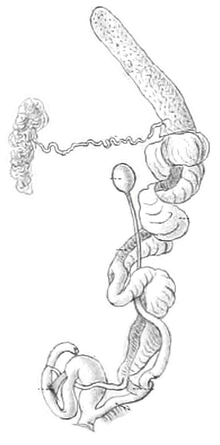
Information concerning the soft anatomy of Amphidromus is widely scattered and fragmentary.[5] The most complete account is that of Arnold Jacobi (1895)[20] on specimens from Great Natuna (Natuna Islands) and Djemadja (Anamba Islands).[5] Unfortunately, although anatomical differences between the two species existed, we have no idea which forms he was dissecting, since, of the two names he used in his paper, Amphidromus chloris is found only in the Philippine Islands and the interruptus phase of Amphidromus perversus is not known from the Natuna Islands.[5] Carl Arend Friedrich Wiegmann (1893, 1898)[21][22] discussed portions of the anatomy of Amphidromus adamsii, Amphidromus porcellanus, Amphidromus contrarius, and Amphidromus sinistralis; Walter Edward Collinge (1901, 1902)[23][24] briefly noted features of Amphidromus palaceus and Amphidromus perakensis (reported as Amphidromus perversus); Haniel (1921)[15] dissected Amphidromus contrarius and Amphidromus reflexilabris; and Bernhard Rensch published a few scattered notes in his various faunistic surveys.[5] A few earlier notes are mentioned in Pilsbry (1900).[5][8]
The long, narrow kidney with reflexed ureter and closed secondary ureter, the penial complex with distinct penis, epiphallus, vas deferens and flagellum, and the basic condition of the nervous and retractor muscle systems all serve to place Amphidromus in the Camaenidae.[5] Apparently, specific differences exist in the penial complex, but present data are insufficient to allow any recognition of species groups through anatomical characters.[5] Laidlaw & Solem (1961)[5] provided no more additional details on anatomy.
Taxonomy
Prior to 1900 the similarity in shape to the South American tree snails had led people to associate Amphidromus with the Bulimulidae.[5] The dissections made by Wiegmann and Jacobi clearly showed that the anatomical features were those of the Asian-Indonesian Camaenidae and that the shell resemblance to bulimulids was a parallelism.[5]
- Subgenera and species
Laidlaw and Solem (1961) recognized 75 species in the genus Amphidromus and another seven names placed under incertae sedis.[5]
In 2010, there are 87 species in the genus Amphidromus.[2][3][4]
Species within the genus Amphidromus are divided into two subgenera and they include:[2]
- subgenus Amphidromus Albers, 1850
Amphidromus perversus (Linnaeus, 1758) is the type species of the genus Amphidromus, by the subsequent designation of Eduard von Martens (1860).[5][25] Species in the subgenus Amphidromus are amphidromine (left-handed and right-handed snails occur in the population) with exceptions: four dextral taxa are: Amphidromus givenchyi, Amphidromus protania, Amphidromus schomburgki dextrochlorus and Amphidromus inversus annamiticus; and one sinistral Amphidromus atricallosus classiaris.[2]
- subgenus Syndromus Pilsbry, 1900[8]
All species of the subgenus Syndromus are sinistral with two exceptions: amphidromine Amphidromus glaucolarynx and dextral Amphidromus kruehni.[4] The type species of the subgenus Syndromus is Amphidromus contrarius Müller, 1774, by the subsequent designation of Adolf Michael Zilch (1960).[5][26]
There have been also used subgenus Goniodromus Bülow, 1905[27] with type Amphidromus büllowi Fruhstorfer, 1905,[5] but its subgeneric status is doubtful.[28]
- Cladogram
Genetic analyses confirmed that the genus Amphidromus is monophyletic.[2] A (simplified) cladogram showing phylogenic relations of (18 analyzed) species of Amphidromus based on partial sequences of 16S rDNA:[2]
| Amphidromus |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
Ecology
Habitat
Amphidromus is a genus of arboreal pulmonate land snails.[5] Information concerning the habits and mode of life of the species of Amphidromus is almost non-existent.[5] They have generally been collected crawling on trees or shrubs.[5]
Feeding habits
The diet of Amphidromus is not known, but Amphidromus atricallosus perakensis probably feeds on microscopic Fungi, lichens or terrestrial algae.[29]
Life cycle
No life history studies have been published and only a few random observations are scattered through the literature.[5] The most important is that of Eugen Paravicini (1921)[30] on egg-laying.[5]
His observations were made on Amphidromus palaceus var. pura at Palimanan, West Java, in October, 1920.[5] Natives brought in two nests with the snails depositing eggs.[5] One snail had folded the exterior leaves of a young bamboo shoot and gummed them together into a pointed cornet.[5] The shoot hung vertically with the narrow end pointed upward and the wide opening below.[5] The upper part of the sack was filled with eggs when collected.[5] The snail descended slowly, rotating around its longitudinal axis, and deposited eggs until the entire cavity was filled.[5] If a crack in the basket exposed eggs to the air, they quickly dried up.[5] Two days after capture, egg-laying was finished and the snail closed the opening by folding over more leaves.[5] Probably four days were spent in egg-laying, since the cavity was half filled at the start of observations.[5] A second nest of similarly folded mango leaves contained 234 eggs.[5]
The volume of eggs in each case greatly exceeded the size of the snail, indicating that the eggs must be encapsulated just before deposition.[5] The capsules were very thin and dried quickly upon any exposure to the air.[5] October marked the start of the rainy season and probably this is the normal breeding period.[5] Eggs of Amphidromus porcellanus were reported by van Benthem Jutting (1950, p. 493)[13] to have started hatching only ten days after being laid.[5] Similar nest-building habits have been reported for other species, but no complete study of a life cycle has been published.[5]
No information were not available on the cycle of activity, longevity, rate of growth, etc. up to 1961,[5] but more information appeared since 1961:
Schilthuizen et al. (2005) described spatial structure of a population of Amphidromus inversus in Malaysia.[31]
Schilthuizen et al. (2007) found, that there is sexual selection in mating with snails of opposite chirality.[32] This means that left-handed snail are mating more often with right-handed snails, than with snails of the same coiling. Additionally there are anatomical adaptations of the spermatophore and of the female part of the reproductive system for these matings success.[32]
Predators
Predators of Amphidromus sp. include red-crowned barbet Megalaima rafflesii.[33] and probably other birds.[29]
Many shells of Amphidromus were found in the den of a rat in Malaysia.[32]
See also
References
This article incorporate public domain text from the reference.[5]
- ↑
 Albers J. C. (1850). Die Heliceen nach natürlicher Verwandtschaft: systematisch georduct: 138.
Albers J. C. (1850). Die Heliceen nach natürlicher Verwandtschaft: systematisch georduct: 138. - 1 2 3 4 5 6 7 8 9 Sutcharit, C.; Asami, T.; Panha, S. (2007). "Evolution of whole-body enantiomorphy in the tree snail genus Amphidromus". Journal of Evolutionary Biology. 20: 661–672. doi:10.1111/j.1420-9101.2006.01246.x. PMC 1920546
 . PMID 17305832.
. PMID 17305832. - 1 2 Chan S.-Y., Tan S.-K. & Abbas J. B. (2008). "On a new species of Amphidromus (Syndromus) (Gastropoda: Pulmonata: Camaenidae) from Rotti Island, Indonesia". Occasional Molluscan Papers 1: 1-5. PDF, Internet Archive
- 1 2 3 Chan S.-Y. & Tan S.-K. (2008). "On a new species of Amphidromus (Syndromus) (Gastropoda: Pulmonata: Camaenidae) from Sumba Island, Indonesia". Occasional Molluscan Papers 1: 6-10. PDF, Internet Archive
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97
 Laidlaw F. F. & Solem A. (1961). "The land snail genus Amphidromus: a synoptic catalogue". Fieldiana Zoology 41(4): 505-720.
Laidlaw F. F. & Solem A. (1961). "The land snail genus Amphidromus: a synoptic catalogue". Fieldiana Zoology 41(4): 505-720. - ↑
 Martens E. von (1867). Die Preussische Expedition nach Ost-Asien. Zoologischer Theil. 2: xii, 447 pp., 22 plates.
Martens E. von (1867). Die Preussische Expedition nach Ost-Asien. Zoologischer Theil. 2: xii, 447 pp., 22 plates. - ↑ Fulton H. (1896). "A list of the species of Amphidromus, Albers, with critical notes and descriptions of some hitherto undescribed species and varieties". Annals and Magazine of Natural History (6)17: 66-94, plates 5-7.
- 1 2 3
 Pilsbry H. A. (1900). Manual of Conchology, structural and systematic, with illustrations of the species. Second series: Pulmonata. Volume 13. Australasian Bulimulidae: Bothriembryon, Placostylus. Helicidae: Amphidromus. 253 pp., 72 plates.
Pilsbry H. A. (1900). Manual of Conchology, structural and systematic, with illustrations of the species. Second series: Pulmonata. Volume 13. Australasian Bulimulidae: Bothriembryon, Placostylus. Helicidae: Amphidromus. 253 pp., 72 plates. - ↑
 Bartsch P. (1917). "The Philippine land snails of the genus Amphidromus". U. S. Nat. Mus. Bull. 100(1), part 1: 1-47, 22 plates.
Bartsch P. (1917). "The Philippine land snails of the genus Amphidromus". U. S. Nat. Mus. Bull. 100(1), part 1: 1-47, 22 plates. - ↑
 Bartsch P. (1918). "The land snails of the genus Amphidromus from the islands of the Palawan Passage". Jour. Washington Acad. Sci. 8(11): 361-367.
Bartsch P. (1918). "The land snails of the genus Amphidromus from the islands of the Palawan Passage". Jour. Washington Acad. Sci. 8(11): 361-367. - ↑
 Bartsch P. (1919). "Critical remarks on Philippine Island land shells". Proc. Biol. Soc. Washington 32: 177-184.
Bartsch P. (1919). "Critical remarks on Philippine Island land shells". Proc. Biol. Soc. Washington 32: 177-184. - ↑ (German) Rensch B. (1932). "Die Mollusken Fauna der Kleinen Sunda-Inseln, Bali, Lombok, Sumbawa, Flores und Sumba". Zool. Jahrb., Syst. 63: 1-130, 3 plates.
- 1 2 van Benthem Jutting T. (1950). "Critical studies of the Javanese pulmonate land-shells of the families Helicarionidae, Pleurodontidae, Fruticicolidae and Streptaxidae". Treubia 20(3): 381-505, 107 figs.
- ↑ van Benthem Jutting T. (1959). "Catalogue of the non-marine Mollusca of Sumatra and of its satellite islands". Beaufortia 7(83): 41-191, 1 plate, 11 figs.
- 1 2 3 (German) Haniel C. (1921). "Variationsstudie an Timoresischen Amphidromus Arten". Zeits. Induct. Abstamm. und Vererbungsl. 25(1-2): 88 pp., 5 plates.
- ↑ (German) Zilch A. M. (1953). "Die Typen und Typoide des Natur-Museums Senckenberg. 10: Mollusca, Pleurodontidae (1)". Archiv für Molluskenkunde 82(4-6): 131-140, plates 22-25.
- 1 2 Solem, A (1983). "First record of Amphidromus from Australia with anatomical note on several species (Mollusca: Pulmonata: Camaenidae)". Records of the Australian Museum. 35 (4): 153–166. doi:10.3853/j.0067-1975.35.1983.315. Archived from the original on 6 July 2011.
- ↑ van Benthem Jutting T. (1932). "On prehistoric shells from Sampoeng Cave (central Java)". Treubia 14(1): 103-108, 5 figs.
- ↑ van Benthem Jutting T. (1937). "Non-marine Mollusca from fossil horizons in Java with special reference to the Trinil fauna. Zool. Meded., 20: 83-180, pis. 4-12.
- ↑ (German) Jacobi A. (1895). "Anatomische Untersuchungen an Malayischen Landschnecken". Arch. Naturg. 61: 293-318, plate 14.
- ↑
 (German) Wiegmann C. A. F. (1863). "Beitrage zur Anatomie der Landschnecken des Indischen Archipels". In: Weber (1863). Zool. Ergeb. Reisen Indischen Arch. 3: 112-259, plates 9-16.
(German) Wiegmann C. A. F. (1863). "Beitrage zur Anatomie der Landschnecken des Indischen Archipels". In: Weber (1863). Zool. Ergeb. Reisen Indischen Arch. 3: 112-259, plates 9-16. - ↑
 (German) Wiegmann C. A. F. (1898). "Landmollusken (Stylommatophoren)". Zootomischer Teil. Abhl. Sencken. Naturf. Gesell. 24(3): 289-557, plates 21-31. 514-527.
(German) Wiegmann C. A. F. (1898). "Landmollusken (Stylommatophoren)". Zootomischer Teil. Abhl. Sencken. Naturf. Gesell. 24(3): 289-557, plates 21-31. 514-527. - ↑ Collinge W. E. (1901). "Note on the anatomy of Amphidromus palaceus, Mouss". Jour. Malac. 8(2): 50-52, plate 4.
- ↑ Collinge W. E. (1902). "On the non-operculated land and fresh-water molluscs collected by members of the "Skeat Expedition" in the Malay Peninsula, 1899-1900". Op. cit. 9(3): 71-95, plates 4-6.
- ↑ von Martens E. (1860). Die Heliceen 2nd ed., p. 184.
- ↑ Zilch A. M. (1960). Gastropoda, Euthyneura. Handb. Palaozool. (6)2(4): 601-834, figs. 2112-2515. page 623.
- ↑ Bülow (1905). Nachr. d. Malak. Gesell. 37: 83.
- ↑ Sutcharit, C. (2005). "TAXONOMIC REVIEW OF THE TREE SNAIL AMPHIDROMUS ALBERS, 1850 (PULMONATA: CAMAENIDAE) IN THAILAND AND ADJACENT AREAS: SUBGENUS AMPHIDROMUS". Journal of Molluscan Studies. 72: 1–30. doi:10.1093/mollus/eyi044.
- 1 2 Lok A. F. S. L. & Tan S. K. (2008). "A review of the Singapore status of the green tree snail, Amphidromus atricallosus perakensis Fulton, 1901 and its biology". Nature in Singapore 1: 225-230. PDF
- ↑ Paravicini E. (1921). "Die Eiablage zweier Javanischer Landschnecken". Archiv für Molluskenkunde 53: 113-116, plate 2.
- ↑ Schilthuizen, M; Scott, B J; Cabanban, A S; Craze, P G (2005). "Population structure and coil dimorphism in a tropical land snail". Heredity. 95 (3): 216–220. doi:10.1038/sj.hdy.6800715. PMID 16077741.
- 1 2 3 Schilthuizen, M.; Craze, P. G.; Cabanban, A. S.; Davison, A.; Stone, J.; Gittenberger, E.; Scott, B. J. (2007). "Sexual selection maintains whole-body chiral dimorphism in snails". Journal of Evolutionary Biology. 20: 1941–1949. doi:10.1111/j.1420-9101.2007.01370.x. PMC 2121153
 . PMID 17714311.
. PMID 17714311. - ↑ Wee J. (2006). "Red-crowned barbet feeding on snail". http://besgroup.talfrynature.com/2006/06/01/red-crowned-barbet-feeding-on-a-snail/. Accessed 9 May 2010.
Further reading
- Craze, Paul G.; Bin Elahan, Berjaya; Schilthuizen, Menno (2006). "Opposite shell-coiling morphs of the tropical land snail Amphidromus martensi show no spatial-scale effects". Ecography. 29: 477–486. doi:10.1111/j.0906-7590.2006.04731.x.
- Dharma, E. (2008). "Penerapan sistem pakar dalam perancangan program identifikasi jenis siput-pohon Amphidromus di Indonesia. Part 1". Berita Solaris. 11 (2): 12–16.
- Dumrongrojwattana P., Mutchacheep S. & Senapin R. (2006). "Identification of 7 Thai arboreal snails genus Amphidromus Alber, 1850 by using morphometrics technique (Pulmonata: Camaenidae)". The Proceeding of 44th Kasetsart University Annual Conference, 30 Jan - 2 Feb 2006, Subject Science: 7 pp. Kasetsart University, Bangkok.
- Goldberg, R. L.; Severns, M. (1997). "Isolation and evolution of the Amphidromus in Nusa Tenggara". American Conchologist. 25 (2): 3–7.
- Panha, S.; Sutcharit, C.; Tongkerd, P.; Burch, J. B. (2001). "Morphogeography of an endemic tree snail genus Amphidromus of Thailand (Pulmonata: Camaenidae)". Of Sea and Shore. 24 (2): 106–113.
- Prasankok, P; Ota, H; Toda, M; Panha, S (2007). "Allozyme variation in the camaenid tree snails Amphidromus atricallosus (Gould, 1843) and A. inversus (Müller, 1774).". Zoological Science. 24 (2): 189–97. doi:10.2108/zsj.24.189. PMID 17409732.
- Severns, M. (2003). "A quick explanation of Amphidromus". Of Sea and Shore. 25 (4): 228–231.
- Severns, M. (2006). "A new species and a new subspecies of Amphidromus from Atauro Island, East Timor (Gastropoda, Pulmonata, Camaenidae)". Basteria. 70: 23–28.
External links
| Wikimedia Commons has media related to Amphidromus. |