Ajoene
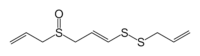 | |
| Identifiers | |
|---|---|
| 92285-01-3 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL122890 |
| ChemSpider | 4533332 |
| PubChem | 5386591 |
| |
| |
| Properties | |
| C9H14OS3 | |
| Molar mass | 234.4 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ajoene /ˈɑːhoʊ.iːn/ is an organosulfur compound found in garlic (Allium sativum). It is a colorless liquid that contains sulfoxide and disulfide functional groups. The name (and pronunciation) is derived from "ajo", the Spanish word for garlic. It is found as a mixture of up to four isomers, which differ in terms of the stereochemistry of central alkene (E- vs Z-) and the chirality of the sulfoxide.
History and biosynthesis
It was first isolated in 1983 by Rafael Apitz-Castro and Mahendra K. Jain (patent US4665088 ) Its basic organic chemistry has been extensively investigated.[1][2][3][4][5][6]
Ajoene is formed from a chemical reaction involving two allicin molecules. The release of allicin occurs when a garlic clove is crushed or finely chopped. Subsequent formation of ajoene occurs when allicin is dissolved in various solvents including edible oils. Ajoene is also found in garlic extract. Ajoene is most stable and most abundant in macerate of garlic (chopped garlic in edible oil).
Medicinal properties
Ajoene has medicinal uses. It functions as an antioxidant by inhibiting the release of superoxide. Ajoene also has antithrombotic (anti-clotting) properties, which helps prevent platelets in the blood from forming blood clots, potentially reducing the risk of heart disease and stroke in humans. Ajoene has shown potential virucidal properties against a number of viruses including vesicular stomatitis, vaccinia, human rhinovirus parainfluenza, and herpes simplex. In the infected cell system of a human immunodeficiency virus (HIV), it is shown to block the integrin-dependent processes.[7][8] Ajoene has broad-spectrum antimicrobial (antibacterial and antifungal) properties.[9][10] For example, Ajoene has been shown to have activity against the human dermatophyte Trichophyton rubrum, the most common cause of tinea pedis, commonly known as Athlete’s Foot.[10][11] The specific mechanism of action is unclear, but is thought to be related to the inhibition of phosphatidylcholine biosynthesis in human dermatophytes.[11] In a randomized study by Ledezma et al. (2000), 70 soldiers from the Venezuelan Armed Forces with KOH or culture proven tinea pedis interdigitalis were randomly distributed into 3 treatment groups: 0.6% ajoene, 1% ajoene, and 1% terbinafine (commercially available as Lamisil AT®) applied twice daily for 1 week.[11] At the 60 day follow up, 72%, 100%, and 94% of the patients treated with 0.6% and 1% ajoene, and 1% terbinafine, respectively, had maintained culture-proven mycologic cure suggesting that short-term topical treatment with ajoene is at least as effective as topical terbinafine for treating tinea pedis.[11] Ajoene has been investigated as an anti-leukemia agent for acute myeloid leukemia therapy.[12] Ajoene has been found to decrease basal-cell carcinoma tumor size by inducing apoptosis[13] while it has also been shown effective in inhibiting tumor cell growth by targeting the microtubule cytoskeleton of such cells and by other mechanisms.[14] Ajoene inhibits genes controlled by quorum sensing.[15]
| Wikimedia Commons has media related to ajoene. |
References
- ↑ Apitz-Cȧstro, R.; Cabrera, S.; Cruz, M.R.; Ledezma, E.; Jain, M.K. (October 1983). "Effects of garlic extract and of three pure components isolated from it on human platelet aggregation, arachidonate metabolism, release reaction and platelet ultrastructure". Thrombosis Research. 32 (2): 155–169. doi:10.1016/0049-3848(83)90027-0. Retrieved Feb 19, 2014.
- ↑ Block E, Ahmad S, Jain MK, Crecely R, Apitz-Castro R, Cruz MR (1984). "(E,Z)-Ajoene: A potent antithrombotic agent from garlic". Journal of the American Chemical Society. 106: 8295–8296. doi:10.1021/ja00338a049.
- ↑ Block, Eric (1985). "The chemistry of garlic and onions". Scientific American. 252 (March): 114–119. doi:10.1038/scientificamerican0385-114. PMID 3975593.
- ↑ Apitz-Castro, R; Escalante, J; Vargas, R; Jain, MK (May 1, 1986). "Ajoene, the antiplatelet principle of garlic, synergistically potentiates the antiaggregatory action of prostacyclin, forskolin, indomethacin and dypiridamole on human platelets.". Thrombosis Research. 42 (3): 303–11. doi:10.1016/0049-3848(86)90259-8. PMID 3520940.
- ↑ Block E, Ahmad S, Catalfamo JL, Jain MK, Apitz-Castro R (1986). "The chemistry of alkyl thiosulfinate esters. 9. Antithrombotic organosulfur compounds from garlic: structural, mechanistic, and synthetic studies". Journal of the American Chemical Society. 108: 7045–7055. doi:10.1021/ja00282a033.
- ↑ Block, Eric (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 978-0-85404-190-9.
- ↑ Weber ND, Andersen DO, North JA, et al. (1992). "In vitro virucidal effects of Allium sativum (garlic) extract and compounds". Planta Med. 58 (5): 417–23. doi:10.1055/s-2006-961504. PMID 1470664.
- ↑ Tatarintsev AV, Vrzhets PV, Ershov DE, et al. (1992). "The ajoene blockade of integrin-dependent processes in an HIV-infected cell system". Vestn Ross Akad Med Nauk (11-12): 6–10. PMID 1284227.
- ↑ Torres J, Romero H (2012). "In vitro antifungal activity of ajoene on five clinical isolates of Histoplasma capsulatum var. capsulatum". Revista Iberoamericana de Micologia. 29 (1): 24–28. doi:10.1016/j.riam.2011.04.001. PMID 21635962.
- 1 2 Ledezma E, Apitz-Castro R (2006). "Ajoene the main active compound of garlic (Allium sativum): a new antifungal agent". Revista Iberoamericana de Micologia. 23 (2): 75–80. doi:10.1016/s1130-1406(06)70017-1. PMID 16854181.
- 1 2 3 4 Ledezma, E.; Marcano, K.; Jorquera, A.; De Sousa L, null; Padilla, M.; Pulgar, M.; Apitz-Castro, R. (2000-11-01). "Efficacy of ajoene in the treatment of tinea pedis: a double-blind and comparative study with terbinafine". Journal of the American Academy of Dermatology. 43 (5 Pt 1): 829–832. ISSN 0190-9622. PMID 11050588.
- ↑ Hassan HT (2004). "Ajoene (natural garlic compound): a new anti-leukaemia agent for AML therapy". Leukemia Research. 28 (7): 667–671. doi:10.1016/j.leukres.2003.10.008. PMID 15158086.
- ↑ Tilli CMLJ; Stavast-Kooy AJW; Vuerstaek JDD; et al. (2003). "The garlic-derived organosulfur component ajoene decreases basal cell carcinoma tumor size by inducing apoptosis". Archives of Dermatological Research. 295 (3): 117–123. doi:10.1007/s00403-003-0404-9. PMID 12756587.
- ↑ Terrasson J, Xu B, Li M, Allart S, Davignon JL, Zhang LH, Wang K, Davrinche C (2007). "Activities of Z-ajoene against tumour and viral spreading in vitro". Fundamental & Clinical Pharmacology. 21 (3): 281–289. doi:10.1111/j.1472-8206.2007.00470.x. PMID 17521297.
- ↑ Givskov M; et al. (2012). "Ajoene, a sulfur rich molecule from garlic, inhibits genes controlled by quorum sensing". Antimicrobial Agents and Chemotherapy. 56 (5): 2314–25. doi:10.1128/AAC.05919-11. PMID 22314537.