Agkistrodon piscivorus
| Agkistrodon piscivorus | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Viperidae |
| Subfamily: | Crotalinae |
| Genus: | Agkistrodon |
| Species: | A. piscivorus |
| Binomial name | |
| Agkistrodon piscivorus (Lacépède, 1789) | |
 | |
| Synonyms | |
|
click to expand
| |
Agkistrodon piscivorus is a venomous snake, a species of pit viper, found in the southeastern United States. Adults are large and capable of delivering a painful and potentially fatal bite. When antagonized, they will stand their ground by coiling their bodies and displaying their fangs.[3] Although their aggression has been exaggerated, individuals may bite when feeling threatened or being handled.[4] This is the world's only semiaquatic viper, usually found in or near water, particularly in slow-moving and shallow lakes, streams, and marshes. The snake is a strong swimmer and will even enter the sea. It has successfully colonized islands off both the Atlantic and Gulf coasts.
The generic name is derived from the Greek words ancistro (hooked) and odon (tooth), and the specific name comes from the Latin piscis (fish) and voro (to eat); thus, the scientific name translates into “hooked-tooth fish-eater”.[5] Common names include variants on water moccasin, swamp moccasin, black moccasin, cottonmouth, gapper, or simply viper.[6] Many of the common names refer to the threat display, where this species will often stand its ground and gape at an intruder, exposing the white lining of its mouth. Three subspecies are currently recognized, including the nominate subspecies described here.[7]
Description



This is the largest species of the genus Agkistrodon. Adults commonly exceed 80 cm (31 in) in length, females grow up to be smaller than males. Total length, per one study of adults, was 65 to 90 cm (26 to 35 in).[8] Average body mass has been found to be 292.5 to 579.6 g (10.32 to 20.44 oz) in males and 201.1 to 254.1 g (7.09 to 8.96 oz) in females.[9][10] Occasionally, individuals may exceed 180 cm (71 in) in length, especially in the eastern part of the range.[11]
Although bigger ones have purportedly been seen in the wild,[12] according to Gloyd and Conant (1990), the largest recorded specimen of A. p. piscivorus was 188 cm (74 in) in length,[13] based on a specimen caught in the Dismal Swamp region and given to the Philadelphia Zoological Garden. It should be noted, however, that this snake had apparently been injured during capture, died several days later and was measured when straight and relaxed.[14] Large specimens can be extremely bulky, with the mass of a specimen of approximately 180 cm (71 in) in length known to attain 4.6 kg (10 lb).[15]
The broad head is distinct from the neck, and the snout is blunt in profile with the rim of the top of the head extending forwards slightly further than the mouth. Substantial cranial plates are present, although the parietal plates are often fragmented, especially towards the rear. A loreal scale is absent. There are six to 9 supralabials and eight to 12 infralabials. At midbody, there are 23–27 rows of dorsal scales.[11] All dorsal scale rows have keels, although those on the lowermost scale rows are weak.[14] In males/females, the ventral scales number 130-145/128-144 and the subcaudals 38-54/36-50. Many of the latter may be divided.[11]
Though the majority of specimens are almost or even totally black, (with the exception of head and facial markings), the color pattern may consist of a brown, gray, tan, yellowish-olive or blackish ground color, which is overlaid with a series of 10–17 dark brown to almost black crossbands. These crossbands, which usually have black edges, are sometimes broken along the dorsal midline to form a series of staggered halfbands on either side of the body. These crossbands are visibly lighter in the center, almost matching the ground color, often contain irregular dark markings, and extend well down onto the ventral scales. The dorsal banding pattern fades with age, so older individuals are an almost uniform olive-brown, grayish-brown or black. The belly is white, yellowish-white or tan, marked with dark spots, and becomes darker posteriorly. The amount of dark pigment on the belly varies from virtually nothing to almost completely black. The head is a more or less uniform brown color, especially in A. p. piscivorus. Subadult specimens may exhibit the same kind of dark, parietal spots characteristic of A. contortrix, but sometimes these are still visible in adults. Eastern populations have a broad, dark, postocular stripe, bordered with pale pigment above and below, that is faint or absent in western populations. The underside of the head is generally whitish, cream or tan.[11]
Juvenile and subadult specimens generally have a more contrasting color pattern, with dark crossbands on a lighter ground color. The ground color is then tan, brown or reddish brown. The tip of the tail is usually yellowish, becoming greenish yellow or greenish in subadults, and then black in adults. On some juveniles, the banding pattern can also be seen on the tail.[11] Young snakes wiggle the tip of their tail to lure prey animals.[16]
This species is often confused with the copperhead, A. contortrix. This is especially true for juveniles, but there are differences. A. piscivorus has broad, dark stripes on the sides of its head that extend back from the eye, whereas A. contortrix has only a thin dark line that divides the pale supralabials from the somewhat darker color of the head. The watersnakes of the genus Nerodia are also similar in appearance, being thick-bodied with large heads, but they have round pupils, no loreal pit, a single anal plate, subcaudal scales that are divided throughout and a distinctive overall color pattern.[11]
Common names
This is a list of common names for Agkistrodon piscivorus, some of which also refer to other species:
- cottonmouth
- black water viper
- black snake
- water pitviper
- worm-tailed pitviper
- water copperhead
- cotton-mouthed snake
- mangrove rattler
- moccasin snake
- water moccasin
- North American water viper
- water rattlesnake
Geographic range

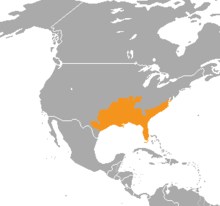
This species is found in the eastern US from the Dismal Swamp in southeast Virginia, south through the Florida peninsula and west to Arkansas, eastern and southern Oklahoma, and western and southern Georgia (excluding Lake Lanier and Lake Allatoona[17]). A few records exist of the species being found along the Rio Grande in Texas, but these are thought to represent disjunct populations, now possibly eradicated. The type locality given is "Carolina", although Schmidt (1953) proposed this be restricted to the area around Charleston, South Carolina.[2]
Campbell and Lamar (2004) mentioned this species as being found in Alabama, Arkansas, Florida, Georgia, Illinois, Indiana, Kentucky, Louisiana, Mississippi, Missouri, North Carolina, Oklahoma, South Carolina, Texas, and Virginia.[11] Maps provided by Campbell and Lamar (2004) and Wright and Wright (1957) also indicate its presence in Western and Middle Tennessee and extreme southeastern Kansas, and limit it to the western part of Kentucky.[6][11]
In Georgia, it is found in the southern half of the state up to a few kilometers north of the fall line with few exceptions. Its range also includes the Ohio River Valley as far north as southern Illinois, and it inhabits many barrier islands off the coasts of the states where it is found.[11]
Conservation status
This species is classified as Least Concern (LC) on the IUCN Red List of Threatened Species (v3.1, 2007).[1] Species are listed as such due to their wide distribution, presumed large population, or because they are unlikely to be declining fast enough to qualify for listing in a more threatened category. When last assessed in 2007, the population trend was stable.[18]
Constant persecution of the species and drainage of wetland habitat prior to development has taken a heavy toll on local populations. Despite this, it remains a common species in many areas.[14][19]
In Indiana, the cottonmouth is listed as an endangered species.[20]
Habitat

This is the most aquatic species of the genus Agkistrodon, and is usually associated with bodies of water, such as creeks, streams, marshes, swamps and the shores of ponds and lakes.[11] The U.S. Navy (1991) describes it as inhabiting swamps, shallow lakes and sluggish streams, but it is usually not found in swift, deep, cool water.[21] Behler and King (1979) list its habitats as including lowland swamps, lakes, rivers, bayheads, sloughs, irrigation ditches, canals, rice fields and small, clear, rocky, mountain streams.[22]
It is also found in brackish water habitats and is sometimes seen swimming in saltwater. It has been much more successful at colonizing Atlantic and Gulf coast barrier islands than the copperhead, A. contortrix. However, even on these islands, it tends to favor freshwater marshes. A study by Dunson and Freda (1985) describes it as not being particularly salt-tolerant.[11]
The snake is not limited to aquatic habitats, however, as Gloyd and Conant (1990) mentioned large specimens have been found more than a mile (1.6 km) from water.[14] In various locations, the species is well-adapted to less moist environments, such as palmetto thickets, pine-palmetto forest, pine woods in East Texas, pine flatwoods in Florida, eastern deciduous dune forest, dune and beach areas, riparian forest and prairies.[11]
Behavior

The aggressiveness of these snakes has been greatly exaggerated. In tests designed to measure the various behavioral responses by wild specimens to encounters with people, 23 of 45 (51%) tried to escape, while 28 of 36 (78%) resorted to threat displays and other defensive tactics. Only when they were picked up with a mechanical hand were they likely to bite.[23]
When sufficiently stressed or threatened, this species engages in a characteristic threat display that includes vibrating its tail and throwing its head back with its mouth open to display the startling white interior,[19] often making a loud hiss while the neck and front part of the body are pulled into an S-shaped position.[24] Many of its common names, including "cottonmouth" and "gaper", refer to this behavior, while its habit of snapping its jaws shut when anything touches its mouth has earned it the name "trap-jaw" in some areas.[25] Other defensive responses can include flattening the body[24] and emitting a strong, pungent secretion from the anal glands located at the base of the tail.[11] This musk may be ejected in thin jets if the snake is sufficiently agitated or restrained. The smell has been likened to that of a billy goat, as well as to a genus of common flood plain weeds, Pluchea, that also have a penetrating odor.[14]
Harmless watersnakes of the genus Nerodia are often mistaken for it. These are also semiaquatic, thick-bodied snakes with large heads that can be aggressive when provoked,[11] but they behave differently. For example, watersnakes usually flee quickly into the water, while A. piscivorus often stands its ground with its threat display. In addition, watersnakes do not vibrate their tails when excited.[26] A. piscivorus usually holds its head at an angle of about 45° when swimming or crawling.[11]
Brown (1973) considered their heavy muscular bodies to be a striking characteristic, stating this made it difficult to hold them for venom extraction owing to their strength.[27]
This species may be active during the day, as well as at night. However, on bright, sunny days, they are usually found coiled or stretched out somewhere in the shade. In the morning and on cool days, they can often be seen basking in the sunlight. They often emerge at sunset to warm themselves on warm ground (i.e., sidewalks, roads) and then become very active throughout the night, when they are usually found swimming or crawling.[11] Contrary to popular belief, they are capable of biting while underwater.[19]
In the north, they hibernate during the winter months. Niell (1947, 1948) made observations in Georgia and noted they were one of the last species to seek shelter, often being found active until the first heavy frosts. At this point, they moved to higher ground and could be found in rotting pine stumps by tearing away the bark. These snakes could be quite active upon discovery and would then attempt to burrow more deeply into the soft wood or escape to the nearest water. In southeastern Virginia, Wood (1954) reported seeing migratory behavior in late October and early November. During a period of three or four days, as many as 50 individuals could be seen swimming across Back Bay from the bayside swamps of the barrier islands to the mainland. He suggested this might have something to do with hibernating habits. In the southern parts of its range, hibernation may be short or omitted altogether.[14]
Feeding
Raymond Ditmars (1912) described this species as "omnicarnivorous". Its diet includes mammals, birds, amphibians, fish, snakes, small turtles and small alligators. Cannibalism has also been reported. Normally, though, the bulk of its diet consists of fish and frogs. On occasion, juvenile specimens feed on invertebrates.[14] Catfish are often eaten, although the sharp spines sometimes cause injuries. Toads of the genus Bufo are apparently avoided.[11]

Many authors have described the prey items taken under natural circumstances. Although fish and frogs are their most common prey, they will eat almost any small vertebrate. Campbell and Lamar (2004) provided an exhaustive list of species that have reportedly been preyed upon by A. piscivorus, including cicadas, caterpillars, land snails (Euglandina rosea), catfish (Ictalurus furcatus), pike (Esox ssp.), sunfishes (Lepomis ssp.), bass (Micropterus ssp.), sirens (Siren spp.), eastern newts (Notophthalmus viridescens), brook salamanders (Eurycea spp.), Ouachita dusky salamanders (Desmognathus brimleyorum), spadefoot toads (Scaphiopus), eastern narrowmouth toads (Gastrophryne carolinensis), northern cricket frogs (Acris crepitans), West Indian treefrogs (Osteopilus septentrionalis), treefrogs (Hyla spp.), true frogs (Rana spp.), green anoles (Anolis carolinensis), skinks (Eumeces spp.), eastern glass lizards (Ophisaurus ventralis), ground skinks (Scincella lateralis), mudsnakes (Farancia abacura), hog-nosed snakes (Heterodon platirhinos), kingsnakes (Lampropeltis spp.), watersnakes (Nerodia spp.), crayfish snakes (Regina spp.), brown snakes (Storeria dekayi), gartersnakes and ribbonsnakes (Thamnophis spp.), other cottonmouths (A. piscivorus), rattlesnakes (Crotalus spp.), common snapping turtles (Chelydra serpentina), mud turtles (Kinosternon spp.), common musk turtles (Sternotherus odoratus), Florida cooters (Pseudemys floridana), sliders (Trachemys scripta), eastern box turtles (Terrapene carolina), Florida softshell turtles (Apalone ferox), baby American alligators (Alligator mississippiensis), wood thrushes (Hylocichla mustelina), chickadees (Parus spp.), cardinals (Cardinalis cardinalis), unidentified passerines, small ducks, juvenile anhingas (Anhinga anhinga), common egrets (Ardea alba), egrets, glossy ibises and their eggs (Plegadis falcinellus), tricolor herons (Egretta tricolor), herons and their eggs, pied-billed grebes (Podilymbus podiceps), short-tailed shrews (Blarina brevicauda), least shrews (Cryptotis parva), southeastern shrews (Sorex longirostris), eastern moles (Scalopus aquaticus), muskrats (Ondatra zibethicus), rice rats (Oryzomys palustris), hispid pocket mice (Perognathus hispidus), black rats (Rattus rattus), squirrels (Sciurus spp.), rabbits (Sylvilagus spp.) and bats.[11]
Fish are captured by cornering them in shallow water, usually against the bank or under logs. They take advantage when bodies of water begin to dry up in the summer or early fall and gorge themselves on the resulting high concentrations of fish and tadpoles. A study by Savitsky (1992) found they were surprisingly unsuccessful at seizing either live or dead fish underwater.[11]
They are opportunistic feeders and will sometimes eat carrion, making them one of the few snakes to do so. Campbell and Lamar (2004) described having seen them feeding on fish heads and viscera that had been thrown into the water from a dock. Heinrich and Studenroth (1996) reported an occasion in which an individual was seen feeding on the butchered remains of a feral hog (Sus scrofa) that had been thrown into Cypress Creek.[11]
Conant (1929) gave a detailed account of the feeding behavior of a captive specimen from South Carolina. When prey was introduced, the snake quickly became attentive and made an attack. Frogs and small birds were seized and held until movement stopped. Larger prey was approached in a more cautious manner; a rapid strike was executed after which the snake would withdraw. In 2.5 years, the snake had accepted three species of frogs, including a large bullfrog; a spotted salamander, water snakes, garter snakes, sparrows, young rats and three species of mice.[14] Brimley (1944) described a captive specimen that ate copperheads (A. contortrix), as well as members of its own species, keeping its fangs embedded in its victims until they had been immobilized.[14]
Young individuals have yellowish or greenish tail tips and engage in caudal luring. The tail tip is wriggled to lure prey, such as frogs and lizards, within striking distance. Wharton (1960) observed captive specimens exhibiting this behavior between 07:20 and 19:40 hours, which suggests it is a daytime activity.[11]
Predators
These snakes are preyed upon by snapping turtles (Chelydra serpentina), American alligators (Alligator mississippiensis), horned owls (Bubo virginianus), eagles, red-shouldered hawks (Buteo lineatus), loggerhead shrikes (Lanius ludovicianus), and large wading birds, such as herons, cranes and egrets.[11][14]
They are also preyed upon by ophiophagous snakes, including their own species. Humphreys (1881) described how a 34-inch (860 mm) specimen was killed and eaten by a 42-inch (1,100 mm) captive kingsnake. On the other hand, Neill (1947) reported captive kingsnakes (Lampropeltis getula) were loath to attack them, being successfully repelled with "body blows".[14] Also called body-bridging, this is a specific defensive behavior against ophiophagous snakes, first observed in certain rattlesnake (Crotalus) species by Klauber (1927), that involves raising a section of the middle of the body above the ground to varying heights. This raised loop may then be held in this position for varying amounts of time, shifted in position, or moved towards the attacker. In the latter case, it is often flipped or thrown vigorously in the direction of the assailant. In A. piscivorus, the loop is raised laterally, with the belly facing towards the attacker.[24]
Reproduction
This species is ovoviviparous, with females giving birth to one to 16 live young and possibly as many as 20. However, litters of six to eight are the most common. Neonates are 22–35 cm in length (excluding runts), with the largest being A. p. conanti and A. p. leucostoma the smallest. If weather conditions are favorable and food is readily available, growth is rapid and females may reproduce at less than three years of age and a total length of as little as 60 cm. The young are born in August or September, while mating may occur during any of the warmer months of the year, at least in certain parts of its range.[14]
Regarding A. p. piscivorus, an early account by Stejneger (1895) described a pair in the Berlin Zoological Garden that mated on January 21, 1873, after which eight neonates were discovered in the cage on July 16 of that year. The young were each 26 cm in length and 1.5 cm thick. They shed for the first time within two weeks, after which they accepted small frogs, but not fish.[14]
Combat behavior between males has been reported on a number of occasions, and is very similar in form to that seen in many other viperid species. An important factor in sexual selection, it allows for the establishment and recognition of dominance as males compete for access to sexually active females.[24]
A few accounts exist that describe females defending their newly born litters. Wharten (1960, 1966) reported several cases where females found near their young stood their ground and considered these to be examples of guarding behavior. Another case was described by Walters and Card (1996) in which a female was found at the entrance of a chamber with seven neonates crawling on or around her. When one of the young was moved a short distance from the chamber, she seemed to be agitated and faced the intruder. Eventually, all of her offspring retreated into the chamber, but the female remained at the entrance, ready to strike.[11]
Facultative parthenogenesis
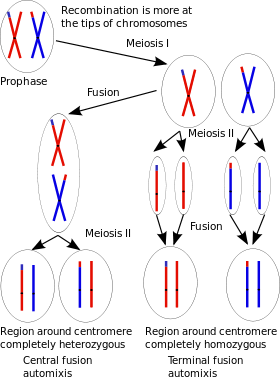
Parthenogenesis is a natural form of reproduction in which growth and development of embryos occur without fertilization. A. piscivorus can reproduce by facultative parthenogenesis, that is, they are capable of switching from a sexual mode of reproduction to an asexual mode.[28] The type of parthenogenesis that likely occurs is “automixis with terminal fusion”, a process in which two terminal products from the same meiosis fuse to form a diploid zygote (see Figure). This process leads to genome wide homozygosity, expression of deleterious recessive alleles and often to developmental failure (inbreeding depression). Both captive-born and wild-born A. piscivorus appear to be capable of this form of parthenogenesis.[28]
Venom
A. piscivorus venom is more toxic than that of A. contortrix (the copperhead) and is rich with tissue-destructive enzymes. It is a powerful cytotoxic venom that destroys tissue. Although deaths are rare, the bite could leave scars and, on occasion, require amputation. Absent an anaphylactic reaction in a bitten individual, however, the venom does not cause systemic reactions in victims and does not contain neurotoxic components that are present in numerous rattlesnake species. Bites can be effectively treated with CroFab antivenom; this serum is derived using venom components from four species of American pit vipers (the eastern and western diamondback rattlesnakes, the Mojave rattlesnake, and the cottonmouth).[29]
Bites from the cottonmouth are relatively frequent in the lower Mississippi River Valley and along the coast of the Gulf of Mexico, although fatalities are rare.[21] Allen and Swindell (1948) compiled a record of A. piscivorus bites in the state of Florida from newspaper accounts and data from the Bureau of Vital Statistics: 1934, eight bites and three fatalities (no further fatalities were recorded after this year); 1935, 10; 1936, 16; 1937, 7; 1938, 6; 1939, 5; 1940, 3; 1941, 6; 1942, 3; 1943, 1; 1944, 3, 1998; 1. Wright and Wright (1957) report having encountered these snakes on countless occasions, often almost stepping on them, but never being bitten. In addition, they heard of no reports of any bites among 400 cypress cutters in the Okefenokee Swamp during the entire summer of 1921. These accounts suggest that the species is not particularly aggressive.[6]
Brown (1973) gave an average venom yield (dried) of 125 mg, with a range of 80–237 mg, along with LD50 values of 4.0, 2.2, 2.7, 3.5, 2.0 mg/kg IV, 4.8, 5.1, 4.0, 5.5, 3.8, 6.8 mg/kg IP and 25.8 mg/kg SC for toxicity.[27] Wolff and Githens (1939) described a 152 cm (60 in) specimen that yielded 3.5 ml of venom during the first extraction and 4.0 ml five weeks later (1.094 grams of dried venom).[6]
Symptoms commonly include ecchymosis and swelling. The pain is generally more severe than bites from the copperhead (A. contortrix) but less so than those from rattlesnakes (Crotalus spp.). The formation of vesicles and bullae is less common than with rattlesnake bites, although necrosis can occur. Myokymia is sometimes reported.[30] On the other hand, the US Navy (1991) states the venom has strong proteolytic activity that can lead to severe tissue destruction.[21]
Subspecies
| Subspecies[7] | Taxon author[7] | Common name[14] | Geographic range[14] |
|---|---|---|---|
| A. p. conanti | Gloyd, 1969 | Florida cottonmouth | The United States, in extreme southern Georgia and virtually all of the state of Florida, including many of the islands off the coast |
| A. p. leucostoma | (Troost, 1836) | Western cottonmouth | The United States, from southern Alabama along coast of the Gulf of Mexico, including many offshore islands, to southeastern and central Texas, and north to Oklahoma, Missouri, Illinois and Indiana |
| A. p. piscivorus | (Lacépède, 1789) | Eastern cottonmouth | The United States in Delmarva Peninsula, the Atlantic Coastal Plain and lower Piedmont of North and South Carolina, including the banks, peninsulas and islands along the Atlantic coast, and west across Georgia |
See also
- List of crotaline species and subspecies
- Crotalinae by common name
- Crotalinae by taxonomic synonyms
- Snakebite
References
- 1 2 G. A. Hammerson (2007). "Agkistrodon piscivorus". IUCN Red List of Threatened Species. Version 2015.2. International Union for Conservation of Nature. Retrieved 16 August 2015.
- 1 2 McDiarmid RW, Campbell JA, Touré T. 1999. Snake Species of the World: A Taxonomic and Geographic Reference, Volume 1. Washington, District of Columbia: Herpetologists' League. 511 pp. ISBN 1-893777-00-6 (series). ISBN 1-893777-01-4 (volume).
- ↑ Cottonmouth Fact Sheet. Smithsonian Institution.
- ↑ Wharton, C.H. (1969). "The cottonmouth moccasin on Sea Horse Key, Florida". Bulletin of the Florida State Museum, Biological Sciences. 14 (3): 227–272.
- ↑ Snakes-uncovered.com : Cottonmouth (Agkistrodon piscivorus)
- 1 2 3 4 Wright AH, Wright AA. 1957. Handbook of Snakes of the United States and Canada. Ithaca and London: Comstock Publishing Associates. (7th printing, 1985). 1,105 pp. (in 2 volumes) ISBN 0-8014-0463-0. (Ancistrodon piscivorus, pp. 916–925, Figures 263–265 , Map 65.)
- 1 2 3 "Agkistrodon piscivorus". Integrated Taxonomic Information System. Retrieved 29 May 2007.
- ↑ Kardong, K. V. (1982). Comparative study of changes in prey capture behavior of the cottonmouth (Agkistrodon piscivorus) and Egyptian cobra (Naja haje). Copeia, 337-343.
- ↑ Vincent, S. E., Herrel, A., & Irschick, D. J. (2004). Sexual dimorphism in head shape and diet in the cottonmouth snake (Agkistrodon piscivorus). Journal of Zoology, 264(1), 53-59.
- ↑ Rainwater, T. R., Reynolds, K. D., Cañas, J. E., Cobb, G. P., Andersonv, T. A., McMurry, S. T., & Smith, P. N. (2005). Organochlorine pesticides and mercury in cottonmouths (Agkistrodon piscivorus) from northeastern Texas, USA. Environmental toxicology and chemistry, 24(3), 665-673.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Campbell JA, Lamar WW. 2004. The Venomous Reptiles of the Western Hemisphere. Ithaca and London: Comstock Publishing Associates. 870 pp. 1500 plates. ISBN 0-8014-4141-2.
- ↑ https://www.youtube.com/watch?v=wKc3EcAQQSQ World's Largest Cottonmouth Snake - Mossy Oak
- ↑ Conant, 1975
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Gloyd HK, Conant R. 1990. Snakes of the Agkistrodon Complex: A Monographic Review. Society for the Study of Amphibians and Reptiles. 614 pp. 52 plates. LCCN 89-50342. ISBN 0-916984-20-6.
- ↑ Roark, A. W. (2003). Comparative genetic analysis in insular and mainland populations of the Florida cottonmouth, Agkistrodon piscivorus conanti (Doctoral dissertation, University of Florida).
- ↑ "Cottonmouth". Smithsonian National Zoological Park. Retrieved 2014-08-10.
- ↑ "Is it a water moccasin?" (PDF). Georgiawildlife.com.
- ↑ 2001 Categories & Criteria (version 3.1) at the IUCN Red List. Accessed 13 September 2007.
- 1 2 3 Mehrtens JM. 1987. Living Snakes of the World in Color. New York: Sterling Publishers. 480 pp. ISBN 0-8069-6460-X.
- ↑ Indiana Legislative Services Agency (2011), "312 IAC 9-5-4: Endangered species of reptiles and amphibians", Indiana Administrative Code, retrieved 28 April 2012
- 1 2 3 U.S. Navy. 1991. Poisonous Snakes of the World. US Govt. New York: Dover Publications Inc. 203 pp. ISBN 0-486-26629-X.
- ↑ Behler JL, King FW. 1979. The Audubon Society Field Guide to North American Reptiles and Amphibians. New York: Alfred A. Knopf. 743 pp. LCCCN 79-2217. ISBN 0-394-50824-6.
- ↑ Gibbons JW, Dorcas ME. 2002. Defensive Behavior of Cottonmouths (Agkistrodon piscivorus) toward Humans. SREL Reprint #2583. Summary at the Savannah River Ecology Laboratory at the University of Georgia. Accessed May 29, 2007.
- 1 2 3 4 Carpenter CC, Gillingham JC. Ritualized Behavior in Agkistrodon and Allied Genera. 523–531. In Gloyd HK, Conant R. 1990. Snakes of the Agkistrodon Complex: A Monographic Review. Society for the Study of Amphibians and Reptiles. 614 pp. 52 plates. LCCN 89-50342. ISBN 0-916984-20-6.
- ↑ Conant R, Bridges W. 1942. What Snake Is That? A Field Guide to the Snakes of the United States East of the Rocky Mountains. New York and London: D. Appleton-Century Company Inc. Frontispiece map + viii + 163 pp. + Plates A–C, 1–32. (Agkistrodon piscivorus pp. 140–141 + Plate 28, Figure 82.)
- ↑ Conant R. 1975. A Field Guide to Reptiles and Amphibians of Eastern and Central North America. Second Edition. First published in 1958. Boston: Houghton Mifflin Company. xviii + 429 pp. + 48 plates. ISBN 0-395-19979-4 (hardcover), ISBN 0-395-19977-8 (paperback). (Agkistrodon piscivorus, pp. 228–230 + Plate 34 + Map 173.)
- 1 2 Brown JH. 1973. Toxicology and Pharmacology of Venoms from Poisonous Snakes. Springfield, Illinois: Charles C. Thomas. 184 pp. LCCCN 73-229. ISBN 0-398-02808-7.
- 1 2 Booth W, Smith CF, Eskridge PH, Hoss SK, Mendelson JR, Schuett GW (2012). "Facultative parthenogenesis discovered in wild vertebrates". Biol. Lett. 8 (6): 983–5. doi:10.1098/rsbl.2012.0666. PMC 3497136
 . PMID 22977071.
. PMID 22977071. - ↑ Agkistrodon piscivorus at Munich AntiVenom INdex (MAVIN). Accessed 18 June 2008.
- ↑ Norris R. 2004. Venom Poisoning in North American Reptiles. In Campbell JA, Lamar WW. 2004. The Venomous Reptiles of the Western Hemisphere. Comstock Publishing Associates, Ithaca and London. 870 pp. 1500 plates. ISBN 0-8014-4141-2.
Further reading
- Allen ER, Swindell D. 1948. "The cottonmouth moccasin of Florida". Herpetologica, 4 (suppl. 1): 1–16.
- Baird SF, Girard C. 1853. Catalogue of North American Reptiles in the Museum of the Smithsonian Institution. Part I.—Serpentes. Washington, District of Columbia: Smithsonian Institution. xvi + 172 pp. (for a discussion of the publication date, see Adler, K. 1963. J. Ohio Herpetol. Soc. 4: 55–57).
- Bonnaterre, P-J. 1790. Ophiologie. pp. 1–76. In Tableau encyclopédique et méthodique des trois règnes de la nature [Encyclopédie Methodique]. Paris, France: Chez Panckoucke, Libraire. i–xliv + 1–76.
- Boulenger GA. 1893–1896. Catalogue of the Snakes in the British Museum (Natural History). Volume I. [1893], Containing the families Typhlopidæ, Glauconiidæ, Boidæ, Ilysiidæ, Uropeltidæ, Xenopeltidæ, Colubridæ Aglyphae, part. xiii + 448 pp. + Plates I-XXVIII. Volume III. [1896], Containing the Colubridæ (Opisthoglyphæ and Proteroglyphæ), Amblycephalidæ, and Viperidæ. xiv + 727 pp. + Plates I-XXV. London: Trustees of the British Museum (Natural History). (Taylor and Francis, printers).
- Brimley CS. 1944. Amphibians and Reptiles of North Carolina. Elon College, North Carolina, Carolina Biol. Supply Co., reprinted from Carolina Tips, 1939–43: 1–63.
- Catesby M. 1743. The natural history of Carolina, Florida and the Bahama Islands: Containing the figures of birds, beasts, fishes, serpents, insects, and plants: Particularly the forest-trees, shrubs, and other plants, not hitherto described, or very incorrectly figured by authors. Together with their descriptions in English and French. To which are added, observation on the air, soil, and waters; With remarks upon agriculture, grain, pulse, roots, &c, To the whole is prefixed a new and correct map of the countries treated of. London, Printed at the expense of the author, 1731–1743: 2 vols. Vol.II: 100 + 200 (appendix).
- Conant R. 1929. Notes on a water moccasin in captivity (Agkistrodon piscivorus) (female). Bull. Antivenin Inst. Amer. 3: 61–64.
- Cope ED. 1860 (dated 1859). Catalogue of the venomous serpents in the museum of the Academy of Natural Sciences of Philadelphia, with notes on the families, genera and species. Proc. Acad. Nat. Sci. Philadelphia 11: 332–347.
- Cope ED. 1875. Check-list of North American Batrachia and Reptiles with a systematic list of higher groups, and an essay on geographical distribution based on specimens contained in the United States National Museum. Washington, D.C.: Government Printing Office. 104 pp.
- Cuvier G. 1829. Le règne animal distribué d'après son organisation, pour servir de base à l'histoire naturelle des animaux det d'introduction à l'anatomie comparée. Tome II, contenant les reptiles, les poissons, les mollusques et les annélidés. Nouvelle édition. Paris: Déterville. xv + 406 pp.
- Daudin FM. 1801–1803. Histoire naturelle, générale et particulière des reptiles: ouvrage faisant suit à l'histoire naturelle générale et particulière, composée par Leclerc de Buffon; et rédigée par C.S. Sonnini, miembre de plusieurs sociétés savantes. 8 vols. Paris: F. Dufart. (for a discussion of the publication date, see F. Harper. 1940. Amer. Midl. Nat. 23: 693).
- Ditmars RL. 1912. The feeding habits of serpents. Zoologica 1: 197–238.
- Duméril A-M-C, Bibron G, Duméril A-H-A. 1854. Erpetologie générale ou histoire naturelle complète des reptiles. Vol. 7. (Parts 1 and 2). Paris: Librarie Encyclopédique de Roret. 1536 pp.
- Dunson WA, Freda J. 1985. Water permeability of the skin of the amphibious snake, Agkistrodon piscivorus. J. Herpetol. 19 (1): 93–98.
- Garman S. 1884 (dated 1883). The reptiles and batrachians of North America. Memoires of the Museum of Comparative Zoology 8 (3): 1–185.
- Garman S. 1890. Notes on Illinois reptiles and amphibians, including several specimens not before recorded from the northern states. Bulletin of the Illinois Natural History Survey 3: 185–190.
- Gloyd HK, Conant R. 1943. A synopsis of the American forms of Agkistrodon (copperheads and moccasins). Bull. Chicago Acad. Sci. 7: 147–170.
- Gray JE. 1842. Synopsis of the species of rattle-snakes, or family of Crotalidae. Zoological Miscellany, London 2: 47–51 (reprinted in 1971 by the Society for the Study of Amphibians and Reptiles).
- Harlan R. 1835. Medical and physical research of original memories in medicine, surgery, physiology, geology, zoology and comparative anatomy. Philadelphia. xxxix + 635 pp.
- Heinrich G, Studenroth KR Jr. 1996. Natural history notes: Agkistrodon piscivorus conanti (Florida cottonmouth). Diet. Herpetol. Rev. 27 (1): 22.
- Higgins SB. 1873. Ophidians, zoological arrangement of the different genera, including varieties known in North and South America, the East Indies, South Africa, and Australia. The poisons, and all that is known of their nature. The galls as antidotes to the snake venom. Pathological, toxicological, and microscopical facts; together with much interesting matter hitherto not published. New York: Boericke & Tafel. 239 pp.
- Holbrook JE. 1838. North American Herpetology; Or, a Description of the Reptiles Inhabiting the United States. Volume 2. Philadelphia, Pennsylvania: J. Dobson: i–iv + 5–125.
- Hubbs B, O'Connor B. 2012. A Guide to the Rattlesnakes and other Venomous Serpents of the United States. Tempe, Arizona: Tricolor Books. 129 pp. ISBN 978-0-9754641-3-7.
- Humphreys JT. 1881. The king snake (Ophibolus sayi) sups on a full grown water moccasin (Ancistrodon piscivorus). Amer. Nat. 15: 561–562.
- Jan G. 1863. Elenco sistematico degli ofidi descritti e disegnati per l'iconografia generale. Milan, Italy: A. Lombardi. vii + 143 pp.
- Klauber LM. 1927. Some observations on the rattlesnakes of the extreme southwest. Bull. Antivenin Inst. Amer. 1 (1): 7–21.
- Lacépède BGE. 1789. Histoire naturelle des quadrupèdes ovipares et des serpentes, vol. 2 Table Méthodique. Paris, France: Hotel de Thou. 527 pp.
- Merrem B. 1820. Versuch eines Systems der Amphibien. Tentamen systematis amphibiorum. Marburg: J.C. Krieger. xv + 191 pp. + 1 plate.
- Niell Jr. WT. 1947. Size and habits of the cottonmouth moccasin. Herpetologica 3: 203–205.
- Niell Jr. WT. 1948. Hibernation of amphibians and reptiles in Richmond County, Georgia. Herpetologica 4: 107–114.
- Schmidt KP. 1953. A check list of North American amphibians and reptiles. Sixth edition. Chicago, Illinois: Amer. Soc. Icthyol. Herpetol. i–viii + 280 pp.
- Shaw G. 1802. General Zoology or Systematic Natural History. Vol. 3. Part 2. Amphibia. London: Thomas Davidson. vi + 313–615.
- Sonnini CS, Latreille PA. 1801. Histoire naturelle des reptiles, avec figures dissinées dápres nature. 4 Vols. Paris (for a discussion of the publication date, see Harper, F. 1940. Amer. Midl. Nat. 23: 692–723).
- Stejneger LH. 1895. The poisonous snakes of North America. Ann. Rept. U.S. Natl. Mus. 1893: 337–487.
- Stewart GD. 1974. Diagnosis of two new American snakes. Baltimore Univ. Comm. (529 N. Howard St. / "an unincorp. free lance organization"), 2:1[1].
- Walters AC, Card W. 1996. Natural history notes: Agkistrodon piscivorus conanti (Florida cottonmouth). Prey. Herpetol. Rev. 27 (4): 203.
- Wharton CH. 1960. Birth and behavior of a brood of cottonmouths, Agkistrodon piscivorus piscivorus, with notes on tail-luring. Herpetologica 16 (2): 125–129.
- Wharton CH. 1966. Reproduction and growth in the cottonmouth, Agkistrodon piscivorus Lacépède, of Cedar Keys, Florida. Copeia 1966 (2): 149–161.
- Wolff NO, Githens TS. 1939. Record venom extraction from water moccasin. Copeia 1939 (1): 52.
- Wood JT. 1954. The distribution of poisonous snakes in Virginia. Virginia Journal of Science 5: 152–167.
- Yarrow HC. 1882. Check list of North American Reptilia and Batrachia, with catalogue of specimens in the United States Museum. Bulletin of the U.S. National Museum 24: 1–249.
External links
| Wikimedia Commons has media related to Agkistrodon piscivorus. |
| Wikispecies has information related to: Agkistrodon piscivorus |
- Agkistrodon piscivorus at the Reptarium.cz Reptile Database. Accessed 7 December 2007.
- Cottonmouth Fact Sheet at Smithsonian National Zoological Park. Accessed 7 December 2007.
- Cottonmouth snake – bites, identification, diet and habitat.
- Water Moccasin Snake * information on identification, range and natural history.
- Video of Agkistrodon piscivorus on YouTube. Accessed 3 July 2008.


