Apocynin
 | |
| Names | |
|---|---|
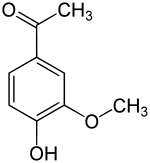
| IUPAC name
1-(4-Hydroxy-3-methoxyphenyl)ethanone | |
| Other names
4-Hydroxy-3-methoxyacetophenone Acetovanillone | |
| Identifiers | |
| 498-02-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:2781 |
| ChEMBL | ChEMBL346919 |
| ChemSpider | 21106900 |
| ECHA InfoCard | 100.007.141 |
| KEGG | C11380 |
| PubChem | 2214 |
| UNII | B6J7B9UDTR |
| |
| |
| Properties | |
| C9H10O3 | |
| Molar mass | 166.17 g/mol |
| Melting point | 115 °C (239 °F; 388 K) |
| Boiling point | 295–300 °C (563–572 °F; 568–573 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Apocynin, also known as acetovanillone, is a natural organic compound structurally related to vanillin. It has been isolated from a variety of plant sources and is being studied for its variety of pharmacological properties.
History
Apocynin was first described by Oswald Schmiedeberg, a German pharmacologist, in 1883 and was first isolated by Horace Finnemore,[1] in 1908,from the root of Canadian hemp (Apocynum cannabinum).[2] At the time, this plant was already used for its known effectiveness against edema and heart problems. In 1971, apocynin was also isolated from Picrorhiza kurroa, a small plant that grows at high altitudes in the western Himalayas. P. kurroa was used for ages as a treatment for liver and heart problems, jaundice, and asthma. In 1990, Simons et al. isolated apocynin to a pharmacologically useful level using an actively guided isolation procedure. Apocynin’s observed anti-inflammatory capabilities proved to be a result of its ability to selectively prevent the formation of free radicals, oxygen ions, and peroxides in the body. Apocynin has since been extensively studied to help determine its disease-fighting capabilities and applications.
Physical properties
Apocynin is a solid with a melting point of 115 °C and the faint odor of vanilla. It is soluble in hot water, alcohol, benzene, chloroform, and ether.
Mode of action
NADPH oxidase is an enzyme that effectively reduces O2 to superoxide (O2–•), which can be used by the immune system to kill bacteria and fungi. Apocynin is an inhibitor of NADPH oxidase activity and thus is effective in preventing the production of the superoxide in human white blood cells or neutrophilic granulocytes. It does not however obstruct the phagocytic or other defense roles of granulocytes. Due to the selectivity of its inhibition, apocynin can be widely used as an inhibitor of NADPH oxidase without interfering in other aspects of the immune system.
Apocynin was used to determine whether ionic activation due to proton flux across the membrane of renal medulla cells was coupled to NADPH oxidase production of superoxide. Apocynin was introduced to the cells and completely blocked the production of superoxide, and was a key component in determining that the proton outflow was responsible for the activation of NADPH oxidase.[3]
The mechanism of action of apocynin is not understood yet. In the experimental studies, apocynin is shown to dimerize and form diapocynin.[4] Although, dipaocynin seems to have beneficial effect in reducing reactive oxygen species and anti-inflammatory properties, it is still yet to be shown as biologically relevant molecule.[5] Biotransformation of apocynin predominantly leads to glycosylated form of apocynin. Another molecule that is shown to form under experimental conditions is nitroapocynin.[6]
Potential use in medical treatments
- Anti-arthritic: Neutrophils are a key component of the pathogenesis of collagen-induced arthritis and in the mechanisms that lead to the start of inflammation of the joints. The action of apocynin reduces the presence of such cells before the inflammation has begun but it is unable to reverse inflammation that is already present.[7]
- Bowel disease: Apocynin treatment in rats has been proven to lessen damage in the colon as well as the enzymatic activity of myeloperoxidase which is associated with inflammation. In addition, apocynin also decreased the number of macrophages and polymorphonuclear leukocytes in the colon.[8]
- Anti-asthmatic: The glucoside of apocynin, androsen, is being investigated for the treatment of asthma for has been shown to prevent bronchial obstruction in guinea pigs. It is believed that the anti-asthmatic quality of apocynin comes from its interference with certain inflammatory processes.[9]
- Atherosclerosis: Apocynin is used in the treatment of atherosclerosis in order to prevent the activity of NADPH oxidase activity, halting the production of reactive oxygen species. In effect, this inhibition stops initiation of disease in the endothelial cells.[9]
- Familial ALS: Apocynin extended the lives of mutant mice and reduced glial cell toxicity of cultured cells lines with a defective Superoxide Dismutase-1 (SOD1) gene—a genetic defect found in some people with hereditary amyotrophic lateral sclerosis (ALS, or Lou Gehrig's disease). Researchers believe the benefit derives from a newly discovered role for SOD1 as a self-regulating redox sensor for NADPH oxidase-derived O2• production. The findings in mice may point to new drug targets for hereditary ALS.[10]
- Erectile dysfuncion: Apocynin is likely to prevent the deleterious effects of reactive oxygen species production in middle-aged rats, thus ameliorating the erectile function.[11][12]
Clinical trials
- It has had a clinical trial in nonsmoking mild asthmatics, it demonstrated an anti-inflammatory action with no adverse events.[13]
- Another trial for COPD completed but does not seem to have published.[14]
References
- ↑ "Acetovanillone". pp. 410–1. in de Stevens, George; Nord, F. F. (1955). "Natural Phenylpropane Derivatives". In Paech, K.; Tracey, M. V. Moderne Methoden der Pflanzenanalyse / Modern Methods of Plant Analysis. Springer-Verlag Berlin Heidelberg. pp. 392–427. doi:10.1007/978-3-642-64958-5_10. ISBN 978-3-642-64958-5.
- ↑ Horace, Finnemore (1908). "The Constituents of Canadian Hemp. Part I. Apocynin". Journal of the Chemical Society. 93 (2): 1513–9. doi:10.1039/ct9089301513. Retrieved 10 April 2014.
- ↑ Li N, Zhang G, Yi FX, Zou AP, Li PL (2005). "Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle". American Journal of Physiology. Renal Physiology. 289 (5): F1048–56. doi:10.1152/ajprenal.00416.2004. PMID 15972387.
- ↑ Luchtefeld, Ron; Dasari, Mina S.; Richards, Kristy M.; Alt, Mikaela L.; Crawford, Clark F. P.; Schleiden, Amanda; Ingram, Jai; Hamidou, Abdel Aziz Amadou; Williams, Angela; Chernovitz, Patricia A.; Sun, Grace Y.; Luo, Rensheng; Smith, Robert E. (2008). "Synthesis of Diapocynin". Journal of Chemical Education. 85 (3): 411. Bibcode:2008JChEd..85..411D. doi:10.1021/ed085p411.
- ↑ Chandasana H, Chhonker YS, Bala V, Prasad YD, Chaitanya TK, Sharma VL, Bhatta RS (2015). "Pharmacokinetic, bioavailability, metabolism and plasma protein binding evaluation of NADPH-oxidase inhibitor apocynin using LC-MS/MS". Journal of Chromatography B. 985: 180–8. doi:10.1016/j.jchromb.2015.01.025. PMID 25682338.
- ↑ Babu S, Raghavamenon AC, Fronczek FR, Uppu RM (2009). "4-Hydr-oxy-3-meth-oxy-5-nitro-aceto-phenone (5-nitro-apocynin)". Acta Crystallographica E. 65 (Pt 9): o2292–3. doi:10.1107/S160053680903390X. PMC 2969931
 . PMID 21577684.
. PMID 21577684. - ↑ 'T Hart BA, Simons JM, Knaan-Shanzer S, Bakker NP, Labadie RP (1990). "Antiarthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin". Free Radical Biology & Medicine. 9 (2): 127–31. doi:10.1016/0891-5849(90)90115-Y. PMID 2172098. INIST:19326251.
- ↑ Palmen, M.J.H.J.; Beukelman, C.J.; Mooij, R.G.M.; Pena A.S.; van Rees, E.P. (1995). "Anti-inflammatory effect of apocynin, a plant-derived NADPH oxidase antagonist, in acute experimental colitis". The Netherlands Journal of Medicine. 47 (2): 41.
- 1 2 Van den Worm E, Beukelman CJ, Van den Berg AJ, Kroes BH, Labadie RP, Van Dijk H (2001). "Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils". European Journal of Pharmacology. 433 (2–3): 225–30. doi:10.1016/S0014-2999(01)01516-3. PMID 11755156.
- ↑ Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schöneich C, Engelhardt JF (2008). "SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model". The Journal of Clinical Investigation. 118 (2): 659–70. doi:10.1172/JCI34060. PMC 2213375
 . PMID 18219391.
. PMID 18219391. - ↑ Silva FH, Mónica FZ, Báu FR, Brugnerotto AF, Priviero FB, Toque HA, Antunes E (2013). "Superoxide anion production by NADPH oxidase plays a major role in erectile dysfunction in middle-aged rats: prevention by antioxidant therapy". The Journal of Sexual Medicine. 10 (4): 960–71. doi:10.1111/jsm.12063. PMID 23347406.
- ↑ Silva FH, Lanaro C, Leiria LO, Rodrigues RL, Davel AP, Claudino MA, Toque HA, Antunes E (2014). "Oxidative stress associated with middle aging leads to sympathetic hyperactivity and downregulation of soluble guanylyl cyclase in corpus cavernosum". American Journal of Physiology. Heart and Circulatory Physiology. 307 (10): H1393–400. doi:10.1152/ajpheart.00708.2013. PMID 25217652.
- ↑ Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, Pawliczak R (2012). "Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics". Experimental Lung Research. 38 (2): 90–9. doi:10.3109/01902148.2011.649823. PMID 22296407.
- ↑ Clinical trial number NCT01402297 for "Hydrogen Peroxide and Nitrite Reduction in Exhaled Breath Condensate of COPD Patients" at ClinicalTrials.gov