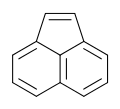
Acenaphthylene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Acenaphthylene | |||
| Systematic IUPAC name
Tricyclo[6.3.1.04,12]dodeca-1(12),2,4,6,8,10-hexaene | |||
| Other names
Cycopenta[de]naphthalene, Acenaphthalene | |||
| Identifiers | |||
| 208-96-8 | |||
| 3D model (Jmol) | Interactive image Interactive image | ||
| ChEBI | CHEBI:33081 | ||
| ChemSpider | 8807 | ||
| ECHA InfoCard | 100.005.380 | ||
| PubChem | 9161 | ||
| UNII | 1Z25C36811 | ||
| |||
| |||
| Properties | |||
| C12H8 | |||
| Molar mass | 152.20 g·mol−1 | ||
| Appearance | Yellow crystals | ||
| Density | 0.8987 g cm−3 | ||
| Melting point | 91.8 °C (197.2 °F; 364.9 K) | ||
| Boiling point | 280 °C (536 °F; 553 K) | ||
| Insoluble | |||
| Solubility in ethanol | very soluble | ||
| Solubility in diethyl ether | very soluble | ||
| Solubility in benzene | very soluble | ||
| Solubility in chloroform | soluble | ||
| Hazards | |||
| R-phrases | R22 R36 R37 R38 | ||
| S-phrases | S26 S36 S37 S39 | ||
| Related compounds | |||
| Related compounds |
acenaphthene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Acenaphthylene is a polycyclic aromatic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a C2H2 unit. It is a yellow solid. Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence.
Occurrence
Acenaphthylene occurs as about 2% of coal tar. It is produced industrially by dehydrogenation of acenaphthene.[1] More than 20% of the carbon in the universe may be associated with PAHs.[2]
Reactions
Hydrogenation gives the more saturated compound acenaphthene.
It functions as a ligand for some organometallic compounds.[3]
References
- ↑ Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke “Hydrocarbons” in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
- ↑ Hoover, Rachel (February 21, 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". NASA. Retrieved February 22, 2014.
- ↑ Yukihiro Motoyama; Chikara Itonaga; Toshiki Ishida; Mikihiro Takasaki; Hideo Nagashima (1925). "Catalytic Reduction of Amides to Amines with Hydrosilanes Using a Triruthenium Cluster as the Catalyst". Org. Synth. 82: 188.; Coll. Vol., 11, p. 1
This article is issued from Wikipedia - version of the 12/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.