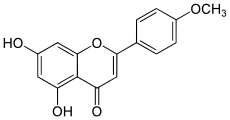
Acacetin
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
5,7-dihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one 5,7-dihydroxy-4′-methoxyflavone 4'-methoxy-5,7-dihydroxyflavone Linarigenin Acacetine Buddleoflavonol Linarisenin 4'-Methoxyapigenin Apigenin 4'-methyl ether 5,7-Dioxy-4'-methoxyflavone | |
| Identifiers | |
| 480-44-4 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:15335 |
| ChEMBL | ChEMBL243664 |
| ChemSpider | 4444099 |
| ECHA InfoCard | 100.006.867 |
| PubChem | 5280442 |
| |
| |
| Properties | |
| C16H12O5 | |
| Molar mass | 284.26 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acacetin is an O-methylated flavone found in Robinia pseudoacacia (black locust), Turnera diffusa (damiana),[1] Betula pendula (silver birch),[2] and in the fern Asplenium normale.[3]
The enzyme apigenin 4'-O-methyltransferase uses S-adenosyl methionine and 5,7,4'-trihydroxyflavone (apigenin) to produce S-adenosylhomocysteine and 4'-methoxy-5,7-dihydroxyflavone (acacetin).
References
- ↑ Zhao, J; Dasmahapatra, AK; Khan, SI; Khan, IA (December 2008). "Anti-aromatase activity of the constituents from damiana (Turnera diffusa)". Journal of Ethnopharmacology. 120 (3): 387–393. doi:10.1016/j.jep.2008.09.016. PMID 18948180.
- ↑ Valkama, E; Salminen, J-P; Koricheva, J; Pihlaja, K. "Changes in Leaf Trichomes and Epicuticular Flavonoids during Leaf Development in Three Birch Taxa". Annals of Botany. 94: 233–242. doi:10.1093/aob/mch131.
- ↑ UmiKalsom, Yusuf; Harborne, Jeffrey B. (1991). "Flavonoid distribution in asplenioid ferns". Pertanika. 14 (3): 297–300.
This article is issued from Wikipedia - version of the 9/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.