AMPA receptor

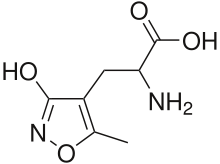
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is a non-NMDA-type ionotropic transmembrane receptor for glutamate that mediates fast synaptic transmission in the central nervous system (CNS). Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen.[1] AMPARs are found in many parts of the brain and are the most commonly found receptor in the nervous system. The AMPA receptor GluA2 (GluR2) tetramer was the first glutamate receptor ion channel to be crystallized.
Structure and function
Subunit composition
AMPARs are composed of four types of subunits, designated as GluR1 (GRIA1), GluR2 (GRIA2), GluR3 (GRIA3), and GluR4, alternatively called GluRA-D2 (GRIA4), which combine to form tetramers.[2][3][4] Most AMPARs are heterotetrameric, consisting of symmetric 'dimer of dimers' of GluR2 and either GluR1, GluR3 or GluR4.[5][6] Dimerization starts in the endoplasmic reticulum with the interaction of N-terminal LIVBP domains, then "zips up" through the ligand-binding domain into the transmembrane ion pore.[6]
The conformation of the subunit protein in the plasma membrane caused controversy for some time. While the amino acid sequence of the subunit indicated that there seemed to be four transmembrane domains (parts of the protein that pass through the plasma membrane), proteins interacting with the subunit indicated that the N-terminus seemed to be extracellular, while the C-terminus seemed to be intracellular. However, if each of the four transmembrane domains went all the way through the plasma membrane, then the two termini would have to be on the same side of the membrane. It was eventually discovered that the second "transmembrane" domain does not in fact cross the membrane at all, but kinks back on itself within the membrane and returns to the intracellular side.[7] When the four subunits of the tetramer come together, this second membranous domain forms the ion-permeable pore of the receptor.
AMPAR subunits differ most in their C-terminal sequence, which determines their interactions with scaffolding proteins. All AMPARs contain PDZ-binding domains, but which PDZ domain they bind to differs. For example, GluR1 binds to SAP97 through SAP97's class I PDZ domain,[8] while GluR2 binds to PICK1[9] and GRIP/ABP. Of note, AMPARs cannot directly bind to the common synaptic protein PSD-95 owing to incompatible PDZ domains, although they do interact with PSD-95 via stargazin (the prototypical member of the TARP family of AMPAR auxiliary subunits).[10]
Phosphorylation of AMPARs can regulate channel localization, conductance, and open probability. GluR1 has four known phosphorylation sites at serine 818 (S818), S831, threonine 840, and S845 (other subunits have similar phosphorylation sites, but GluR1 has been the most extensively studied). S818 is phosphorylated by protein kinase C, and is necessary for long-term potentiation (LTP; for GluR1's role in LTP, see below).[11] S831 is phosphorylated by CaMKII and PKC during LTP, which helps deliver GluR1-containing AMPAR to the synapse,[12] and increases their single channel conductance.[13] The T840 site was more recently discovered, and has been implicated in LTD.[14] Finally, S845 is phosphorylated by PKA which regulates its open probability.[15]
Ion channel function
Each AMPAR has four sites to which an agonist (such as glutamate) can bind, one for each subunit.[5] The binding site is believed to be formed by the N-terminal tail and the extracellular loop between transmembrane domains three and four.[16] When an agonist binds, these two loops move towards each other, opening the pore. The channel opens when two sites are occupied,[17] and increases its current as more binding sites are occupied.[18] Once open, the channel may undergo rapid desensitization, stopping the current. The mechanism of desensitization is believed to be due to a small change in angle of one of the parts of the binding site, closing the pore.[19] AMPARs open and close quickly (1ms), and are thus responsible for most of the fast excitatory synaptic transmission in the central nervous system.[17] The AMPAR's permeability to calcium and other cations, such as sodium and potassium, is governed by the GluR2 subunit. If an AMPAR lacks a GluR2 subunit, then it will be permeable to sodium, potassium, and calcium. The presence of a GluR2 subunit will almost always render the channel impermeable to calcium. This is determined by post-transcriptional modification — RNA editing — of the Q-to-R editing site of the GluR2 mRNA. Here, A→I editing alters the uncharged amino acid glutamine (Q) to the positively charged arginine (R) in the receptor's ion channel. The positively charged amino acid at the critical point makes it energetically unfavourable for calcium to enter the cell through the pore. Almost all of the GluR2 subunits in CNS are edited to the GluR2(R) form. This means that the principal ions gated by AMPARs are sodium and potassium, distinguishing AMPARs from NMDA receptors (the other main ionotropic glutamate receptors in the brain), which also permit calcium influx. Both AMPA and NMDA receptors, however, have an equilibrium potential near 0 mV. The prevention of calcium entry into the cell on activation of GluR2-containing AMPARs is proposed to guard against excitotoxicity.[20]
The subunit composition of the AMPAR is also important for the way this receptor is modulated. If an AMPAR lacks GluR2 subunits, then it is susceptible to being blocked in a voltage-dependent manner by a class of molecules called polyamines. Thus, when the neuron is at a depolarized membrane potential, polyamines will block the AMPAR channel more strongly, preventing the flux of potassium ions through the channel pore. GluR2-lacking AMPARs are, thus, said to have an inwardly rectifying I/V curve, which means that they pass less outward current than inward current.
Alongside RNA editing, alternative splicing allows a range of functional AMPA receptor subunits beyond what is encoded in the genome. In other words, although one gene (GRIA1–GRIA4) is encoded for each subunit (GluR1–GluR4), splicing after transcription from DNA allows some exons to be translated interchangeably, leading to several functionally different subunits from each gene.
The flip/flop sequence is one such interchangeable exon. A 38-amino acid sequence found prior to (i.e., before the N-terminus of) the fourth membranous domain in all four AMPAR subunits, it determines the speed of desensitisation[21] of the receptor and also the speed at which the receptor is resensitised[22] and the rate of channel closing.[23] The flip form is present in prenatal AMPA receptors and gives a sustained current in response to glutamate activation.[24]
Synaptic plasticity
AMPA receptors (AMPAR) are both glutamate receptors and cation channels that are integral to plasticity and synaptic transmission at many postsynaptic membranes. One of the most widely and thoroughly investigated forms of plasticity in the nervous system is known as long-term potentiation, or LTP. There are two necessary components of LTP: presynaptic glutamate release and postsynaptic depolarization. Therefore, LTP can be induced experimentally in a paired electrophysiological recording when a presynaptic cell is stimulated to release glutamate on a postsynaptic cell that is depolarized. The typical LTP induction protocol involves a “tetanus” stimulation, which is a 100 Hz stimulation for 1 second. When one applies this protocol to a pair of cells, one will see a sustained increase of the amplitude of the EPSP following tetanus. This response is interesting since it is thought to be the physiological correlate for learning and memory in the cell. In fact, it was recently shown that, following a single paired-avoidance paradigm in mice, LTP could be recorded in some hippocampal synapses in vivo.[25]
The molecular basis for LTP has been extensively studied, and AMPARs have been shown to play an integral role in the process. Both GluR1 and GluR2 play an important role in synaptic plasticity. It is now known that the underlying physiological correlate for the increase in EPSP size is a postsynaptic upregulation of AMPARs at the membrane,[26] which is accomplished through the interactions of AMPARs with many cellular proteins.
The simplest explanation for LTP is as follows (see the long-term potentiation article for a much more detailed account). Glutamate binds to postsynaptic AMPARs and another glutamate receptor, the NMDA receptor (NMDAR). Ligand binding causes the AMPARs to open, and Na+ flows into the postsynaptic cell, resulting in a depolarization. NMDARs, on the other hand, do not open directly because their pores are occluded at resting membrane potential by Mg2+ ions. NMDARs can open only when a depolarization from the AMPAR activation leads to repulsion of the Mg2+ cation out into the extracellular space, allowing the pore to pass current. Unlike AMPARs, however, NMDARs are permeable to both Na+ and Ca2+. The Ca2+ that enters the cell triggers the upregulation of AMPARs to the membrane, which results in a long-lasting increase in EPSP size underlying LTP. The calcium entry also phosphorylates CaMKII, which phosphorylates AMPARs, increasing their single-channel conductance.
AMPA receptor trafficking
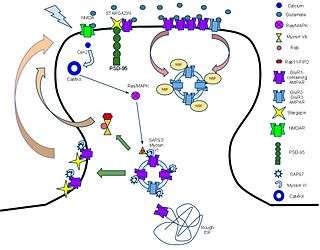
Molecular and signaling response to LTP-inducing stimuli
The mechanism for LTP has long been a topic of debate, but, recently, mechanisms have come to some consensus. AMPARs play a key role in this process, as one of the key indicators of LTP induction is the increase in the ratio of AMPAR to NMDARs following high-frequency stimulation. The idea is that AMPARs are trafficked from the dendrite into the synapse and incorporated through some series of signaling cascades.
AMPARs are initially regulated at the transcriptional level at their 5’ promoter regions. There is significant evidence pointing towards the transcriptional control of AMPA receptors in longer-term memory through cAMP response element-binding protein (CREB) and Mitogen-activated protein kinases (MAPK).[27] Messages are translated on the rough endoplasmic reticulum (rough ER) and modified there. Subunit compositions are determined at the time of modification at the rough ER.[9] After post-ER processing in the golgi apparatus, AMPARs are released into the perisynaptic membrane as a reserve waiting for the LTP process to be initiated.
The first key step in the process following glutamate binding to NMDARs is the influx of calcium through the NMDA receptors and the resultant activation of Ca2+/calmodulin-dependent protein kinase (CaMKII).[28] Blocking either this influx or the activation of CaMKII prevents LTP, showing that these are necessary mechanisms for LTP.[29] In addition, profusion of CaMKII into a synapse causes LTP, showing that it is a causal and sufficient mechanism.[30]
CaMKII has multiple modes of activation to cause the incorporation of AMPA receptors into the perisynaptic membrane. CAMKII enzyme is eventually responsible for the development of the actin cytoskeleton of neuronal cells and, eventually, for the dendrite and axon development (synaptic plasticity).[31] The first is direct phosphorylation of synaptic-associated protein 97(SAP97).[32] First, SAP-97 and Myosin-VI, a motor protein, are bound as a complex to the C-terminus of AMPARs. Following phosphorylation by CaMKII, the complex moves into the perisynaptic membrane.[33] The second mode of activation is through the MAPK pathway. CaMKII activates the Ras proteins, which go on to activate p42/44 MAPK, which drives AMPAR insertion directly into the perisynaptic membrane.[34]
AMPA receptor trafficking to the PSD in response to LTP
Once AMPA receptors are transported to the perisynaptic region through PKA or SAP97 phosphorylation, receptors are then trafficked to the postsynaptic density (PSD). However, this process of trafficking to the PSD still remains controversial. One possibility is that, during LTP, there is lateral movement of AMPA receptors from perisynpatic sites directly to the PSD.[35] Another possibility is that exocytosis of intracellular vesicles is responsible for AMPA trafficking to the PSD directly.[36] Recent evidence suggests that both of these processes are happening after an LTP stimulus; however, only the lateral movement of AMPA receptors from the perisynaptic region enhances the number of AMPA receptors at the PSD.[37] The exact mechanism responsible for lateral movement of AMPA receptors to the PSD remains to be discovered; however, research has discovered several essential proteins for AMPA receptor trafficking. For example, overexpression of SAP97 leads to increased AMPA receptor trafficking to synapses.[38] In addition to influencing synaptic localization, SAP97 has also been found to influence AMPA receptor conductance in response to glutamate.[39] Myosin proteins are calcium sensitive motor proteins that have also been found to be essential for AMPA receptor trafficking. Disruption of myosin Vb interaction with Rab11 and Rab11-FIP2 blocks spine growth and AMPA receptor trafficking.[40] Therefore, it is possible that myosin may drive the lateral movement of AMPA receptors in the perisynpatic region to the PSD. Transmembrane AMPA regulatory proteins (TARPs) are a family proteins that associate with AMPA receptors and control their trafficking and conductance.[41] CACNG2 (Stargazin) is one such protein and is found to bind AMPA receptors in the perisynaptic and postsynaptic regions.[42] The role of stargazin in trafficking between the perisynaptic and postsynaptic regions remains unclear; however, stargazin is essential for immobilizing AMPA receptors in the PSD by interacting with PSD-95.[43] PSD-95 stabilizes AMPA receptors to the synapse and disruption of the stargazin-PSD-95 interaction suppressed synaptic transmission.[44]
Constitutive trafficking and changes in subunit composition
AMPA receptors are continuously being trafficked (endocytosed, recycled, and reinserted) into and out of the plasma membrane. Recycling endosomes within the dendritic spine contain pools of AMPA receptors for such synaptic reinsertion.[45] Two distinct pathways exist for the trafficking of AMPA receptors: a regulated pathway and a constitutive pathway.[46][47]
In the regulated pathway, GluR1-containing AMPA receptors are trafficked to the synapse in an activity-dependent manner, stimulated by NMDA receptor activation.[12] Under basal conditions, the regulated pathway is essentially inactive, being transiently activated only upon the induction of long-term potentiation.[45][46] This pathway is responsible for synaptic strengthening and the initial formation of new memories.[48]
In the constitutive pathway, GluR1-lacking AMPA receptors, usually GluR2-GluR3 heteromeric receptors, replace the GluR1-containing receptors in a one-for-one, activity-independent manner,[49][50] preserving the total number of AMPA receptors in the synapse.[45][46] This pathway is responsible for the maintenance of new memories, sustaining the transient changes resulting from the regulated pathway. Under basal conditions, this pathway is routinely active, as it is necessary also for the replacement of damaged receptors.
The GluR1 and GluR4 subunits consist of a long carboxy (C)-tail, whereas the GluR2 and GluR3 subunits consist of a short carboxy-tail. The two pathways are governed by interactions between the C termini of the AMPA receptor subunits and synaptic compounds and proteins. Long C-tails prevent GluR1/4 receptors from being inserted directly into the postsynaptic density zone (PSDZ) in the absence of activity, whereas the short C-tails of GluR2/3 receptors allow them to be inserted directly into the PSDZ.[35][51] The GluR2 C terminus interacts with and binds to N-ethylmaleimide sensitive fusion protein,[52][53][54] which allows for the rapid insertion of GluR2-containing AMPA receptors at the synapse.[55] In addition, GluR2/3 subunits are more stably tethered to the synapse than GluR1 subunits.[56][57][58]
LTD-induced endocytosis of AMPA receptors
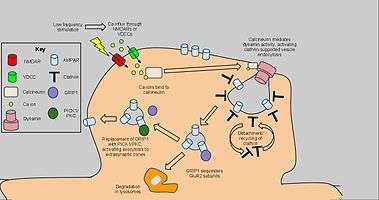
Long-term depression enacts mechanisms to decrease AMPA receptor density in selected dendritic spines, dependent on clathrin and calcineurin and distinct from that of constitutive AMPAR trafficking. The starting signal for AMPAR endocytosis is an NMDAR-dependent calcium influx from low-frequency stimulation, which in turn activates protein phosphatases PP1 and calcineurin. However, AMPAR endocytosis has also been activated by voltage-dependent calcium channels, agonism of AMPA receptors, and administration of insulin, suggesting general calcium influx as the cause of AMPAR endocytosis.[59] Blockage of PP1 did not prevent AMPAR endocytosis, but antagonist application to calcineurin led to significant inhibition of this process.[60]
Calcineurin interacts with an endocytotic complex at the postsynaptic zone, explaining its effects on LTD.[61] The complex, consisting of a clathrin-coated pit underneath a section of AMPAR-containing plasma membrane and interacting proteins, is the direct mechanism for reduction of AMPARs, in particular GluR2/GluR3 subunit-containing receptors, in the synapse. Interactions from calcineurin activate dynamin GTPase activity, allowing the clathrin pit to excise itself from the cell membrane and become a cytoplasmic vesicle.[62] Once the clathrin coat detaches, other proteins can interact directly with the AMPARs using PDZ carboxyl tail domains; for example, glutamate receptor-interacting protein 1 (GRIP1) has been implicated in intracellular sequestration of AMPARs.[63] Intracellular AMPARs are subsequently sorted for degradation by lysosomes or recycling to the cell membrane.[64] For the latter, PICK1 and PKC can displace GRIP1 to return AMPARs to the surface, reversing the effects of endocytosis and LTD when appropriate.[65] Nevertheless, the highlighted calcium-dependent, dynamin-mediated mechanism above has been implicated as a key component of LTD and as such may have applications to further behavioral research.[66]
Role in Seizures
AMPA receptors play a key role in the generation and spread of epileptic seizures.[67] Kainic acid, a convulsant that is widely used in epilepsy research induces seizures, in part, via activation of AMPA receptors [68]
Molecular target for epilepsy therapy
The noncompetitive AMPA receptor antagonists talampanel and perampanel have been demonstrated to have activity in the treatment of adults with partial seizures,[69][70] indicating that AMPA receptor antagonists represent a potential target for the treatment of epilepsy.[71] [72] Perampanel (trade name: Fycompa) received Marketing Authorisation Approval by the European Commission for the treatment of partial epilepsy on July 27, 2012. The drug was approved in the United States by the Food and Drug Administration (FDA) on October 22, 2012. As has been the case for most recently developed AEDs including pregabalin, lacosamide and ezogabine, the FDA recommended that perampanel be classified by the Drug Enforcement Administration (DEA) as a scheduled drug. It has been designated as a Schedule 3 controlled substance.
Ligands
Agonists
Positive allosteric modulators
- Aniracetam
- Cyclothiazide
- CX-516
- CX-546
- CX-614
- derivative 11r[74]
- CX-691
- CX-717
- IDRA-21
- LY-392,098
- LY-404,187
- LY-451,395
- LY-451,646
- LY-503,430 [75][76]
- Org 26576
- Oxiracetam
- PEPA
- Piracetam
- Pramiracetam
- Sunifiram
- Tianeptine,[77] a rapid-acting antidepressant.
- Unifiram
Antagonists
- CNQX
- Kynurenic acid - endogenous ligand
- NBQX - selective for AMPA receptor over kainate receptor
- Tezampanel
- Perampanel
Negative allosteric modulators
- Ethanol
- GYKI-52,466
- GYKI-53,655
- Perampanel
- Talampanel
See also
References
- ↑ Honore T, Lauridsen J, Krogsgaard-Larsen P (1982). "The binding of [3H]AMPA, a structural analogue of glutamic acid, to rat brain membranes". Journal of Neurochemistry. 38 (1): 173–178. doi:10.1111/j.1471-4159.1982.tb10868.x. PMID 6125564.
- ↑ "Glutamate receptors: Structures and functions. University of Bristol Centre for Synaptic Plasticity.". Archived from the original on 15 September 2007. Retrieved 2007-09-02.
- ↑ Shi SH, Hayashi Y, Petralia RS, et al. (1999). "Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation". Science. 284 (5421): 1811–6. doi:10.1126/science.284.5421.1811. PMID 10364548.
- ↑ Song I, Huganir RL (2002). "Regulation of AMPA receptors during synaptic plasticity". Trends Neurosci. 25 (11): 578–88. doi:10.1016/S0166-2236(02)02270-1. PMID 12392933.
- 1 2 Mayer, M. L. (2005). "Glutamate receptor ion channels". Current Opinion in Neurobiology. 15 (3): 282–288. doi:10.1016/j.conb.2005.05.004. PMID 15919192.
- 1 2 Greger IH, Ziff EB, Penn AC (August 2007). "Molecular determinants of AMPA receptor subunit assembly". Trends Neurosci. 30 (8): 407–16. doi:10.1016/j.tins.2007.06.005. PMID 17629578.
- ↑ Hollmann M, Maron C, Heinemann S (1994). "N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1". Neuron. 13 (6): 1331–43. doi:10.1016/0896-6273(94)90419-7. PMID 7993626.
- ↑ Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW (July 1998). "SAP97 is associated with GluR1 subunit". J. Biol. Chem. 273 (31): 19518–24. doi:10.1074/jbc.273.31.19518. PMID 9677374.
- 1 2 Greger IH, Khatri L, Ziff EB (May 2002). "RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum". Neuron. 34 (5): 759–72. doi:10.1016/S0896-6273(02)00693-1. PMID 12062022.
- ↑ Bats C, Groc L, Choquet D (2007). "The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking.". Neuron. 53 (5): 719–34. doi:10.1016/j.neuron.2007.01.030. PMID 17329211.
- ↑ Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R (July 2006). "Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1". Neuron. 51 (2): 213–25. doi:10.1016/j.neuron.2006.06.013. PMID 16846856.
- 1 2 Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (March 2000). "Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction". Science. 287 (5461): 2262–7. doi:10.1126/science.287.5461.2262. PMID 10731148.
- ↑ Derkach V, Barria A, Soderling TR (March 1999). "Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors". Proc. Natl. Acad. Sci. U.S.A. 96 (6): 3269–74. doi:10.1073/pnas.96.6.3269. PMC 15931
 . PMID 10077673.
. PMID 10077673. - ↑ Delgado JY, Coba M, Anderson CN, et al. (November 2007). "NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840". J. Neurosci. 27 (48): 13210–21. doi:10.1523/JNEUROSCI.3056-07.2007. PMC 2851143
 . PMID 18045915.
. PMID 18045915. - ↑ Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF (January 2000). "Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase". J. Neurosci. 20 (1): 89–102. PMID 10627585.
- ↑ Armstrong N, Sun Y, Chen GQ, Gouaux E (October 1998). "Structure of a glutamate-receptor ligand-binding core in complex with kainate". Nature. 395 (6705): 913–7. doi:10.1038/27692. PMID 9804426.
- 1 2 Platt SR (2007). "The role of glutamate in central nervous system health and disease--a review". Vet. J. 173 (2): 278–86. doi:10.1016/j.tvjl.2005.11.007. PMID 16376594.
- ↑ Rosenmund C, Stern-Bach Y, Stevens CF (June 1998). "The tetrameric structure of a glutamate receptor channel". Science. 280 (5369): 1596–9. doi:10.1126/science.280.5369.1596. PMID 9616121.
- ↑ Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E (October 2006). "Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor". Cell. 127 (1): 85–97. doi:10.1016/j.cell.2006.08.037. PMID 17018279.
- ↑ Kim DY, Kim SH, Choi HB, Min C, Gwag BJ (2001). "High abundance of GluR1 mRNA and reduced Q/R editing of GluR2 mRNA in individual NADPH-diaphorase neurons". Mol. Cell. Neurosci. 17 (6): 1025–33. doi:10.1006/mcne.2001.0988. PMID 11414791.
- ↑ Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP (1994). "A molecular determinant for submillisecond desensitization in glutamate receptors". Science. 266 (5187): 1059–62. doi:10.1126/science.7973663. PMID 7973663.
- ↑ Sommer B, Keinänen K, Verdoorn TA, et al. (1990). "Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS". Science. 249 (4976): 1580–5. doi:10.1126/science.1699275. PMID 1699275.
- ↑ Pei W, Huang Z, Niu L (2007). "GluR3 flip and flop: differences in channel opening kinetics". Biochemistry. 46 (7): 2027–36. doi:10.1021/bi062213s. PMID 17256974.
- ↑ GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT–PCR) study, Eastwood et al., Molecular Brain Research Vol44, Iss1, Feb1997, Pg92-98
- ↑ Whitlock JR, Heynen AJ, Shuler MG, Bear MF (2006). "Learning induces long-term potentiation in the hippocampus". Science. 313 (5790): 1093–7. doi:10.1126/science.1128134. PMID 16931756.
- ↑ Maren S, Tocco G, Standley S, Baudry M, Thompson RF (1993). "Postsynaptic factors in the expression of long-term potentiation (LTP): increased glutamate receptor binding following LTP induction in vivo". Proceedings of the National Academy of Sciences. 90 (20): 9654–8. doi:10.1073/pnas.90.20.9654. PMID 8415757.
- ↑ Perkinton, M. S.; Sihra, T. S.; Williams, R. J. (Jul 15, 1999). "Ca(2+)-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons". The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 19 (14): 5861–5874. ISSN 1529-2401. PMID 10407026. Retrieved 2015-04-21.
- ↑ Fukunaga K, Stoppini L, Miyamoto E, Muller D (April 1993). "Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II". J. Biol. Chem. 268 (11): 7863–7. PMID 8385124.
- ↑ Lisman J, Schulman H, Cline H (March 2002). "The molecular basis of CaMKII function in synaptic and behavioural memory". Nat. Rev. Neurosci. 3 (3): 175–90. doi:10.1038/nrn753. PMID 11994750.
- ↑ Mammen AL, Kameyama K, Roche KW, Huganir RL (December 1997). "Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II". J. Biol. Chem. 272 (51): 32528–33. doi:10.1074/jbc.272.51.32528. PMID 9405465.
- ↑ Ebert, D. H., & Greenberg, M. E. (2013). Activity-dependent neuronal signalling and autism spectrum disorder. Nature, 493(7432), 327-337.
- ↑ Mauceri D, Cattabeni F, Di Luca M, Gardoni F (May 2004). "Calcium/calmodulin-dependent protein kinase II phosphorylation drives synapse-associated protein 97 into spines". J. Biol. Chem. 279 (22): 23813–21. doi:10.1074/jbc.M402796200. PMID 15044483.
- ↑ Wu H, Nash JE, Zamorano P, Garner CC (August 2002). "Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking". J. Biol. Chem. 277 (34): 30928–34. doi:10.1074/jbc.M203735200. PMID 12050163.
- ↑ Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R (August 2002). "Ras and Rap control AMPA receptor trafficking during synaptic plasticity". Cell. 110 (4): 443–55. doi:10.1016/S0092-8674(02)00897-8. PMID 12202034.
- 1 2 Borgdorff AJ, Choquet D (June 2002). "Regulation of AMPA receptor lateral movements". Nature. 417 (6889): 649–53. doi:10.1038/nature00780. PMID 12050666.
- ↑ Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD (September 2004). "Recycling endosomes supply AMPA receptors for LTP". Science. 305 (5692): 1972–5. doi:10.1126/science.1102026. PMID 15448273.
- ↑ Makino H, Malinow R (November 2009). "AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis". Neuron. 64 (3): 381–90. doi:10.1016/j.neuron.2009.08.035. PMC 2999463
 . PMID 19914186.
. PMID 19914186. - ↑ Howard MA, Elias GM, Elias LA, Swat W, Nicoll RA (February 2010). "The role of SAP97 in synaptic glutamate receptor dynamics". Proc. Natl. Acad. Sci. U.S.A. 107 (8): 3805–10. doi:10.1073/pnas.0914422107. PMC 2840522
 . PMID 20133708.
. PMID 20133708. - ↑ Waites CL, Specht CG, Härtel K, Leal-Ortiz S, Genoux D, Li D, Drisdel RC, Jeyifous O, Cheyne JE, Green WN, Montgomery JM, Garner CC (April 2009). "Synaptic SAP97 isoforms regulate AMPA receptor dynamics and access to presynaptic glutamate". J. Neurosci. 29 (14): 4332–45. doi:10.1523/JNEUROSCI.4431-08.2009. PMC 3230533
 . PMID 19357261.
. PMID 19357261. - ↑ Wang Z, Edwards JG, Riley N, Provance DW, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD (October 2008). "Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity". Cell. 135 (3): 535–48. doi:10.1016/j.cell.2008.09.057. PMC 2585749
 . PMID 18984164.
. PMID 18984164. - ↑ Nicoll RA, Tomita S, Bredt DS (March 2006). "Auxiliary subunits assist AMPA-type glutamate receptors". Science. 311 (5765): 1253–6. doi:10.1126/science.1123339. PMID 16513974.
- ↑ Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS (May 2003). "Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins". J. Cell Biol. 161 (4): 805–16. doi:10.1083/jcb.200212116. PMC 2199354
 . PMID 12771129.
. PMID 12771129. - ↑ Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA (2000). "Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms". Nature. 408 (6815): 936–43. doi:10.1038/35050030. PMID 11140673.
- ↑ Bats C, Groc L, Choquet D (March 2007). "The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking". Neuron. 53 (5): 719–34. doi:10.1016/j.neuron.2007.01.030. PMID 17329211.
- 1 2 3 Shepherd JD, Huganir RL (2007). "The cell biology of synaptic plasticity: AMPA receptor trafficking". Annu. Rev. Cell Dev. Biol. 23: 613–43. doi:10.1146/annurev.cellbio.23.090506.123516. PMID 17506699.
- 1 2 3 Malinow R, Mainen ZF, Hayashi Y (June 2000). "LTP mechanisms: from silence to four-lane traffic". Curr. Opin. Neurobiol. 10 (3): 352–7. doi:10.1016/S0959-4388(00)00099-4. PMID 10851179.
- ↑ Malenka RC (November 2003). "Synaptic plasticity and AMPA receptor trafficking". Ann. N. Y. Acad. Sci. 1003: 1–11. doi:10.1196/annals.1300.001. PMID 14684431.
- ↑ Kessels HW, Malinow R (February 2009). "Synaptic AMPA receptor plasticity and behavior". Neuron. 61 (3): 340–50. doi:10.1016/j.neuron.2009.01.015. PMC 3917551
 . PMID 19217372.
. PMID 19217372. - ↑ McCormack SG, Stornetta RL, Zhu JJ (April 2006). "Synaptic AMPA receptor exchange maintains bidirectional plasticity". Neuron. 50 (1): 75–88. doi:10.1016/j.neuron.2006.02.027. PMID 16600857.
- ↑ Zhu JJ, Esteban JA, Hayashi Y, Malinow R (November 2000). "Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity". Nat. Neurosci. 3 (11): 1098–106. doi:10.1038/80614. PMID 11036266.
- ↑ Passafaro M, Piëch V, Sheng M (September 2001). "Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons". Nat. Neurosci. 4 (9): 917–26. doi:10.1038/nn0901-917. PMID 11528423.
- ↑ Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL (August 1998). "Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors". Neuron. 21 (2): 393–400. doi:10.1016/S0896-6273(00)80548-6. PMID 9728920.
- ↑ Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB (July 1998). "The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs". Neuron. 21 (1): 99–110. doi:10.1016/S0896-6273(00)80518-8. PMID 9697855.
- ↑ Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM (July 1998). "NSF binding to GluR2 regulates synaptic transmission". Neuron. 21 (1): 87–97. doi:10.1016/S0896-6273(00)80517-6. PMID 9697854.
- ↑ Beretta F, Sala C, Saglietti L, Hirling H, Sheng M, Passafaro M (April 2005). "NSF interaction is important for direct insertion of GluR2 at synaptic sites". Mol. Cell. Neurosci. 28 (4): 650–60. doi:10.1016/j.mcn.2004.11.008. PMID 15797712.
- ↑ Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y (June 2008). "Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins". Neuron. 58 (5): 749–62. doi:10.1016/j.neuron.2008.04.011. PMC 2446609
 . PMID 18549786.
. PMID 18549786. - ↑ Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M, Passafaro M (May 2007). "Extracellular interactions between GluR2 and N-cadherin in spine regulation". Neuron. 54 (3): 461–77. doi:10.1016/j.neuron.2007.04.012. PMID 17481398.
- ↑ Silverman JB, Restituito S, Lu W, Lee-Edwards L, Khatri L, Ziff EB (August 2007). "Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes". J. Neurosci. 27 (32): 8505–16. doi:10.1523/JNEUROSCI.1395-07.2007. PMID 17687028.
- ↑ Carroll RC, Beattie EC, Xia H, Lüscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M (November 1999). "Dynamin-dependent endocytosis of ionotropic glutamate receptors". Proc. Natl. Acad. Sci. U.S.A. 96 (24): 14112–7. doi:10.1073/pnas.96.24.14112. PMC 24199
 . PMID 10570207.
. PMID 10570207. - ↑ Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC (December 2000). "Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD". Nat. Neurosci. 3 (12): 1291–300. doi:10.1038/81823. PMID 11100150.
- ↑ Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, Snyder SH (September 1999). "The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis". J. Biol. Chem. 274 (37): 25963–6. doi:10.1074/jbc.274.37.25963. PMID 10473536.
- ↑ Jung N, Haucke V (September 2007). "Clathrin-mediated endocytosis at synapses". Traffic. 8 (9): 1129–36. doi:10.1111/j.1600-0854.2007.00595.x. PMID 17547698.
- ↑ Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT (December 2000). "PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses". Neuron. 28 (3): 873–86. doi:10.1016/S0896-6273(00)00160-4. PMID 11163273.
- ↑ Ehlers, MD (2000). "Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting". Neuron. 28 (2): 511–25. doi:10.1016/S0896-6273(00)00129-X. PMID 11144360.
- ↑ Lu W, Ziff EB (August 2005). "PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking". Neuron. 47 (3): 407–21. doi:10.1016/j.neuron.2005.07.006. PMID 16055064.
- ↑ Yang, YT (2008). "Probing the role of AMPAR endocytosis and long-term depression in behavioural sensitization: relevance to treatment of brain disorders, including drug addiction". British Journal of Pharmacology. 153 (S1): S389–S395. doi:10.1038/sj.bjp.0707616.
- ↑ Rogawski MA (2013). "AMPA receptors as a molecular target in epilepsy therapy". Acta Neurol. Scand. Suppl. 127 (197): 9–18. doi:10.1111/ane.12099. PMID 23480151.
- ↑ Fritsch B, Reis J, Gasior M, Kaminski RM, Rogawski MA (April 2014). "Role of GluK1 Kainate Receptors in Seizures, Epileptic Discharges, and Epileptogenesis". J. Neurosci. 34 (17): 5765–75. doi:10.1523/JNEUROSCI.5307-13.2014. PMID 24760837.
- ↑ Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T (January 2007). "Progress report on new antiepileptic drugs: a summary of the Eighth Eilat Conference (EILAT VIII)". Epilepsy Res. 73 (1): 1–52. doi:10.1016/j.eplepsyres.2006.10.008. PMID 17158031.
- ↑ French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, Kumar D, Rogawski MA (August 2012). "Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304". Neurology. 79 (6): 589–96. doi:10.1212/WNL.0b013e3182635735. PMC 3413761
 . PMID 22843280.
. PMID 22843280. - ↑ Rogawski MA (March 2011). "Revisiting AMPA receptors as an antiepileptic drug target". Epilepsy Curr. 11 (2): 56–63. doi:10.5698/1535-7511-11.2.56. PMC 3117497
 . PMID 21686307.
. PMID 21686307. - ↑ Sakai F, Igarashi H, Suzuki S, Tazaki Y (1989). "Cerebral blood flow and cerebral hematocrit in patients with cerebral ischemia measured by single-photon emission computed tomography". Acta Neurol. Scand. Suppl. 127: 9–13. PMID 2631521.
- ↑ "'Major' Ketamine Discovery May Lead to New Antidepressants". Retrieved 2016-05-17.
- ↑ Mueller R, Rachwal S, Tedder ME, Li YX, Zhong S, Hampson A, Ulas J, Varney M, Nielsson L, Rogers G (July 2011). "Substituted benzoxazinones as potent positive allosteric AMPA receptor modulators: part II". Bioorg. Med. Chem. Lett. 21 (13): 3927–30. doi:10.1016/j.bmcl.2011.05.024. PMID 21636273.
- ↑ Murray TK, Whalley K, Robinson CS, et al. (2003). "LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson's disease". J. Pharmacol. Exp. Ther. 306 (2): 752–62. doi:10.1124/jpet.103.049445. PMID 12730350.
- ↑ O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES (2004). "AMPA receptor potentiators for the treatment of CNS disorders". Curr Drug Targets CNS Neurol Disord. 3 (3): 181–94. doi:10.2174/1568007043337508. PMID 15180479.
- ↑ McEwen, BS; Chattarji, S; Diamond, DM; Jay, TM; Reagan, LP; Svenningsson, P; Fuchs, E (March 2010). "The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation.". Molecular Psychiatry. 15 (3): 237–49. doi:10.1038/mp.2009.80. PMC 2902200
 . PMID 19704408.
. PMID 19704408.