AB5 toxin
The AB5 toxins are six-component protein complexes secreted by certain pathogenic bacteria known to cause human diseases such as cholera, dysentery, and hemolytic-uremic syndrome. One component is known as the A subunit, and the remaining five components make up the B subunit. All of these toxins share a similar structure and mechanism for entering targeted host cells. The B subunit is responsible for binding to receptors to open up a pathway for the A subunit to enter the cell. The A subunit is then able to use its catalytic machinery to take over the host cell's regular functions.[1][2]
Families
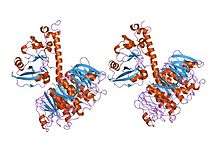
_PDB_1r4q.png)
There are four main families of the AB5 toxin. These families are characterized by the sequence of their A subunit, as well as their catalytic ability.[4]
Cholera toxin
This family is also known as Ct or Ctx, and includes the heat-labile enterotoxin family, known as LT.[5] Cholera toxin’s discovery is credited by many to Dr. Sambhu Nath De. He conducted his research in Calcutta (now Kolkata) making his discovery in 1959, although it was first purified by Robert Koch in 1883. Cholera toxin is an infectious toxin composed of a protein complex that is secreted by the bacterium Vibrio cholerae.[6] Some symptoms of this toxin include chronic and widespread watery diarrhea and dehydration that, in some cases, leads to death.
Pertussis toxin
This family is also known as Ptx and contains the toxin responsible for whooping cough. Pertussis toxin is secreted by the gram-negative bacterium, Bordetella pertussis. Whooping cough is very contagious and cases are slowly increasing in the United States despite vaccination.[7] Symptoms include paroxysmal cough with whooping and even vomiting.[8] The bacterium Bordetella pertussis was first identified as the cause of whooping cough and isolated by Jules Bordet and Octave Gengou in France in 1900.[9]
Shiga toxin
Shiga toxin is an infectious disease caused by the rod shaped Shigella dysenteriae as well as Escherichia coli (STEC), and is also known as Stx. Contaminated food and drinks are the source of infection and how this toxin is spread.[10] Symptoms include abdominal pain as well as watery diarrhea. Severe life-threatening cases are characterized by hemorrhagic colitis (HC).[11] The discovery of shiga toxin is credited to Dr. Kiyoshi Shiga in 1898.
Subtilase cytotoxin
This family is also known as SubAB[4] and was discovered during the 1990s.[12] It produced by strains of STEC that do not have the locus of enterocyte effacement (LEE),[13] and is known to cause hemolytic-uremic syndrome (HUS). It is called a subtilase cytotoxin because its A subunit sequence is similar to that of a subtilase-like serine protease in Bacillus anthracis. Some symptoms caused by this toxin are a decrease in platelet count in the blood or thrombocytopenia, an increase in white blood cell count or leukocytosis, and renal cell damage.[14]
Structure
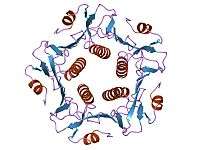
A complete AB5 toxin complex contains six protein units. Five units are similar or identical in structure and they comprise the B subunit. The last protein unit is unique and is known as the A subunit.
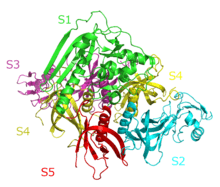
A subunit
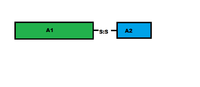
The A subunit of an AB5 toxin is the portion responsible for catalysis of specific targets. For Shiga toxin family, the A subunit hosts a Trypsin-sensitive region which gives out two fragmented domains when cleaved. This region has not been confirmed for the other AB5 toxin families as yet.[2] In general, the two domains of the A subunit, named A1 and A2, are linked by a disulfide bond. Domain A1 (approximately 22kDa in cholera toxin or heat labile enterotoxins) is the part of the toxin responsible for its toxic effects. Domain A2 (approximately 5kDa in cholera toxin or heat labile enterotoxin) provides a non-covalent linkage to the B subunit through the B subunit's central pore.[5] The A1 chain for cholera toxin catalyzes the transfer of ADP-ribose from Nicotinamide adenine dinucleotide(NAD) to arginine or other guanidine compounds by utilizing ADP-ribosylation factors (ARFs). In the absence of arginine or simple guanidino compounds, the toxin mediated NAD+ nucleosidase (NADase) activity proceeds using water as a nucleophile.[15]
B subunit
The B subunits form a five-membered or pentameric ring, where one end of the A subunit goes into and is held. This B subunit ring is also capable of binding to a receptor on the surface of the host cell.[16] Without the B subunits, the A subunit has no way of attaching to or entering the cell, and thus no way to exert its toxic effect. Cholera toxin, shiga toxin, and SubAB toxin all have B subunits that are made up of five identical protein components, meaning that their B subunits are homopentamers. Pertussis toxin is different where its pentameric ring is made up of four different protein components, where one of the components is repeated to form a heteropentamer.[5]
Mechanisms
Cholera toxin, pertussis toxin, and shiga toxin all have their targets in the cytosol of the cell. After their B subunit binds to receptors on the cell surface, the toxin is enveloped by the cell and transported inside either through clathrin-dependent endocytosis or clathrin-independent endocytosis.[17]
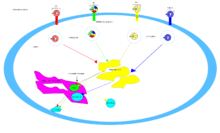
For the cholera toxin, the principal glycolipid receptor for the cholera toxin is ganglioside GM1.[16] After endocytosis to the golgi apparatus, the toxin is redirected to the endoplasmic reticulum.[5] In order for the A subunit to reach its target, a disulfide bond between the A1 and A2 domain must be broken. This breakage is catalyzed by a protein disulfide isomerase[18] that is in the endoplasmic reticulum. Following separation, the A1 domain unfolds and is redirected back to the cytosol where it refolds[5] and catalyzes ADP-ribosylation of certain G protein alpha subunits. In doing so, the downstream effects of the G protein signal transduction pathway is disrupted[4] by activating adenylate cyclase.[16] This causes a higher concentration of cAMP in the cell, which disrupts the regulation of ion transport mechanisms.[5]
The pertussis toxin does not have a specific receptor, and binds to sialylated glycoproteins.[12] After endocytosis, pertussis toxin's mechanism is the same as cholera toxin.
The main receptor for the shiga toxin is globotriaosylceramide or Gb3.[19] Shiga toxin is also brought to the golgi apparatus before being directed to the endoplasmic reticulum for PDI to cleave the disulfide bond. Shiga toxin's A subunit is then brought back into the cytosol and inhibits eukaryotic protein synthesis with its RNA N-glycosidase activity[4] by cleaving a specific adenine base on 28S ribosomal RNA[5] that will ultimately cause cell death.
SubAB's target is in the endoplasmic reticulum of the cell and is brought into the cell through clathrin-mediated endocytosis.[16] The glycan receptor for SubAB usually ends with an α2-3-linked N-Glycolylneuraminic acid (Neu5Gc).[12] SubAB has an A subunit where it acts as a serine protease and cleaves Bip/GRP78, an endoplasmic reticulum chaperone.[4] The cleavage of this chaperone causes cellular stress through protein inhibition,[13] and consequently death of the cell.[5]
Medical uses
Cancer treatment
B subunits of the AB5 toxins have the affinity towards binding glycan which some type of tumors seem to possess making it an easy target. One example is that of StxB which specifically binds with CD77 which shows expression on the surface of cancerous cells such as colon, pancreas, breast, and many more. Once StxB targets a cancerous cell, it delivers the A subunit of the toxin which eventually kills the cancerous cell.[5]
Yet another method is by using ER stress-inducing drugs which have been tested in mice to show positive synergistic responses. This is accomplished through fusion of epidermal growth factor(EGF) with SubAB's A subunit. Cancer cells that express receptors for EGF will then experience SubAB toxicity.[20]
Vaccines
Another use of AB5 toxins is using members of the LT family as adjuvants. This allows the toxin to promote immunological responses such as IgG2a, IgA, and Th17 to fight for instance gastric Helicobacter pylori infection when a vaccine is given.[21][22]
In addition to some of these AB5 toxins being used to create vaccines to prevent bacterial infection, they are also being researched to work as a conjugate to prevent viral infections. For example, systemic immunization along with co-administered intra-nasal delivery of virus-cholera toxin conjugate vaccine induced a virus-specific antibody response and showed some degree of protection to the upper respiratory tract from Sendai virus.[23]
Recent areas of research
New advancements in biotechnological experimental methods such as the use of Bessel beam plane illumination microscopy and FRET-based sensor molecules can better demonstrate dynamic structures of gap junction plaques. For these experiments, different types of AB5 toxins can be used to induce the fast formation of tCDR in E.Coli cells. The response can then be recorded using cAMP concentration fluctuations in gap junction-coupled cells using FRET-based sensor constructs. Research suggests that CDRs could perhaps be linked with rapid rearrangement of lipids and protein in connexin channels within the gap junction plaques. This can further help us understand the signaling cascade that follows a cellular loss of K+ when exposed to bacterial infection.[24][25]
The SubAB toxin has been seen to demonstrate specificity to a binding protein, BiP. This characteristic has been utilized to study the role of the cellular BiP itself, along with Endoplasmic-reticulum-associated degradation in stressed HeLa cells.[5]
See also
References
- ↑ Le Nours, J.; Paton, A. W.; Byres, E.; Troy, S.; Herdman, B. P.; Johnson, M. D.; Paton, J. C.; Rossjohn, J.; Beddoe, T. (6 August 2013). "Structural Basis of Subtilase Cytotoxin SubAB Assembly". Journal of Biological Chemistry. 288 (38): 27505–27516. doi:10.1074/jbc.M113.462622.
- 1 2 Middlebrook, JL; Dorland, RB (Sep 1984). "Bacterial toxins: cellular mechanisms of action.". Microbiological reviews. 48 (3): 199–221. PMC 373009
 . PMID 6436655.
. PMID 6436655. - ↑ Locht, C; Antoine, R (1995). "A proposed mechanism of ADP-ribosylation catalyzed by the pertussis toxin S1 subunit.". Biochimie. 77 (5): 333–40. doi:10.1016/0300-9084(96)88143-0. PMID 8527486.
- 1 2 3 4 5 Wang, H; Paton, JC; Herdman, BP; Rogers, TJ; Beddoe, T; Paton, AW (Mar 2013). "The B subunit of an AB5 toxin produced by Salmonella enterica serovar Typhi up-regulates chemokines, cytokines, and adhesion molecules in human macrophage, colonic epithelial, and brain microvascular endothelial cell lines.". Infection and immunity. 81 (3): 673–83. doi:10.1128/IAI.01043-12. PMC 3584882
 . PMID 23250951.
. PMID 23250951. - 1 2 3 4 5 6 7 8 9 10 Beddoe, Travis; Paton, Adrienne W.; Le Nours, Jérôme; Rossjohn, Jamie; Paton, James C. (July 2010). "Structure, biological functions and applications of the AB5 toxins". Trends in Biochemical Sciences. 35 (7): 411–418. doi:10.1016/j.tibs.2010.02.003.
- ↑ Bharati, K. Ganguly, N.K. (February 2011) Cholera toxin: A paradigm of a multifunctional protein. Indian J. Med Res; 113(2):179-187 PMC3089049
- ↑ Millen, S.H Schneider, O.D. Miller, W.E. Monaco, J.J. Weise, A.A. (September 2013) Pertussis Toxin B-Pentamer Mediates Intercellular Transfer of Membrane Proteins and Lipids. PLoS One 2013;8(9)e72885 PMC3760862
- ↑ Carbonetti NH (2010) Pertussis toxin and adenylate cyclase toxin: key virulence factors ofBordetella pertussis and cell biology tools. Future Microbiol 5: 455–469. PMC2851156
- ↑ Guiso N. 2009.Bordetella pertussis and pertussis vaccines. Clin. Infect. Dis. 49:1565–1569
- ↑ Faruque SM, Chowdhury N, Khan R, Hasan MR, Nahar J, Islam MJ, et al. Shigella dysenteriaetype 1-specific bacteriophage from environmental waters in Bangladesh. Appl Environ Microbiol.2003;69:7028–31. doi: 10.1128/AEM.69.12.7028-7031.2003 PMC310026
- ↑ Beutin L, Miko A, Krause G, Pries K, Haby S et al. (2007) Identification of Human-Pathogenic Strains of Shiga Toxin-Producing Escherichia coli from Food by a Combination of Serotyping and Molecular Typing of Shiga Toxin Genes. Appl Environ Microbiol 73: 4769-4775.10.1128/AEM.00873-07 PubMed: 17557838
- 1 2 3 Paton, AW; Paton, JC (Feb 1, 2010). "Escherichia coli Subtilase Cytotoxin.". Toxins. 2 (2): 215–228. doi:10.3390/toxins2020215. PMC 2943149
 . PMID 20871837.
. PMID 20871837. - 1 2 Michelacci, V.; Tozzoli, R.; Caprioli, A.; Martínez, R.; Scheutz, F.; Grande, L.; Sánchez, S.; Morabito, S.; Allerberger, F. (2013). "A new pathogenicity island carrying an allelic variant of the Subtilase cytotoxin is common among Shiga toxin producing of human and ovine origin". Clinical Microbiology and Infection. 19 (3): E149–E156. doi:10.1111/1469-0691.12122.
- ↑ Wang, Hui; Paton, James C.; Paton, Adrienne W. (October 2007). "Pathologic Changes in Mice Induced by Subtilase Cytotoxin, a Potent New AB Toxin That Targets the Endoplasmic Reticulum". The Journal of Infectious Diseases. 196 (7): 1093–1101. doi:10.1086/521364.
- ↑ Gutkind, edited by Toren Finkel, J. Silvio (2003). Signal Transduction and Human Disease. Hoboken, NJ: John Wiley & Sons. ISBN 0471448370.
- 1 2 3 4 Lencer W, Saslowsky D (2005). "Raft trafficking of AB5 subunit bacterial toxins". Biochim Biophys Acta. 1746 (3): 314–21. doi:10.1016/j.bbamcr.2005.07.007. PMID 16153723.
- ↑ Smith, Richard D.; Willett, Rose; Kudlyk, Tetyana; Pokrovskaya, Irina; Paton, Adrienne W.; Paton, James C.; Lupashin, Vladimir V. (October 2009). "The COG Complex, Rab6 and COPI Define a Novel Golgi Retrograde Trafficking Pathway that is Exploited by SubAB Toxin". Traffic. 10 (10): 1502–1517. doi:10.1111/j.1600-0854.2009.00965.x.
- ↑ Teter, Ken (10 December 2013). "Toxin Instability and Its Role in Toxin Translocation from the Endoplasmic Reticulum to the Cytosol". Biomolecules. 3 (4): 997–1029. doi:10.3390/biom3040997. (PDI)
- ↑ Thorpe, C. M. (1 May 2004). "Shiga Toxin--Producing Escherichia coli Infection". Clinical Infectious Diseases. 38 (9): 1298–1303. doi:10.1086/383473.
- ↑ Backer, JM; Krivoshein, AV; Hamby, CV; Pizzonia, J; Gilbert, KS; Ray, YS; Brand, H; Paton, AW; Paton, JC; Backer, MV (Nov 2009). "Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells.". Neoplasia (New York, N.Y.). 11 (11): 1165–73. PMC 2767218
 . PMID 19881952.
. PMID 19881952. - ↑ Norton, E. B.; Lawson, L. B.; Mahdi, Z.; Freytag, L. C.; Clements, J. D. (23 April 2012). "The A Subunit of Escherichia coli Heat-Labile Enterotoxin Functions as a Mucosal Adjuvant and Promotes IgG2a, IgA, and Th17 Responses to Vaccine Antigens". Infection and Immunity. 80 (7): 2426–2435. doi:10.1128/IAI.00181-12.
- ↑ Weltzin, R; Guy, B; Thomas WD, Jr; Giannasca, PJ; Monath, TP (May 2000). "Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection.". Infection and Immunity. 68 (5): 2775–82. doi:10.1128/iai.68.5.2775-2782.2000. PMC 97487
 . PMID 10768972.
. PMID 10768972. - ↑ Liang, XP; Lamm, ME; Nedrud, JG (Sep 1, 1988). "Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus.". Journal of immunology (Baltimore, Md. : 1950). 141 (5): 1495–501. PMID 2842395.
- ↑ Majoul, IV; Gao, L; Betzig, E; Onichtchouk, D; Butkevich, E; Kozlov, Y; Bukauskas, F; Bennett, MV; Lippincott-Schwartz, J; Duden, R (Oct 29, 2013). "Fast structural responses of gap junction membrane domains to AB5 toxins.". Proceedings of the National Academy of Sciences of the United States of America. 110 (44): E4125–33. doi:10.1073/pnas.1315850110. PMC 3816413
 . PMID 24133139.
. PMID 24133139. - ↑ Börner, S; Schwede, F; Schlipp, A; Berisha, F; Calebiro, D; Lohse, MJ; Nikolaev, VO (Apr 2011). "FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells.". Nature protocols. 6 (4): 427–38. doi:10.1038/nprot.2010.198. PMID 21412271.
External links
- Bacterial AB5 Toxins
- Held, Paul. "An Introduction to Fluorescence Resonance Energy Transfer (FRET) Technology and its Application in Bioscience". BioTek Instruments, Inc.