4-O-Methylhonokiol
 | |
| Names | |
|---|---|
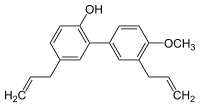
| IUPAC name
2-(4-Methoxy-3-prop-2-enylphenyl)-4-prop-2-enylphenol | |
| Other names
3,5′-Diallyl-2′-hydroxy-4-methoxybiphenyl | |
| Identifiers | |
| 68592-15-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 136692 |
| PubChem | 155160 |
| |
| |
| Properties | |
| C19H20O2 | |
| Molar mass | 280.37 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-O-Methylhonokiol is a neolignan, a type of phenolic compounds. It is found in the bark of Magnolia grandiflora[1] and in M. virginiana flowers.[2]
4-O-Methylhonokiol is a potent CB2 receptor ligand (Ki = 50 nM), showing a unique inverse agonism and partial agonism via different pathways (cAMP and Ca2+) and potently inhibits osteoclastogenesis.[3] 4-O-Methylhonokiol further attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway.[4] The different neuroprotective and anti-Alzheimer Disease effects reported in rodent models may be mediated via CB2 receptors, providing that the compound should be orally bioavailable to the brain.[5] It was shown that 4-O-methylhonokiol activates CB2 receptors and also inhibits the oxygenation of the major endocannabinoid 2-AG via COX-2 in a substrate-selective manner, thus leading to potential synergistic effects at CB receptors.[6] The same study also provided data that this natural product can readily pass the blood–brain barrier, using LC-MS/MS.
References
- ↑ Clark, Alice M.; El-Feraly, Arouk S.; Li, Wen-Shyong (1981). "Antimicrobial activity of phenolic constituents ofmagnolia grandiflora L". Journal of Pharmaceutical Sciences. 70 (8): 951–2. doi:10.1002/jps.2600700833. PMID 7310672.
- ↑ Chandra, Amitabh; Nair, Muraleedharan (2007). "Supercritical Carbon Dioxide Extraction and Quantification of Bioactive Neolignans fromMagnolia virginianaFlowers". Planta Medica. 61 (2): 192–5. doi:10.1055/s-2006-958051. PMID 7753933.
- ↑ Schuehly, Paredes; Kleyer, Huefner; Anavi-Goffer, Raduner; Altmann, Gertsch (2011). "Mechanisms of osteoclastogenesis inhibition by a novel class of biphenyl-type cannabinoid CB(2) receptor inverse agonists.". Chemistry and Biology. 26 (18): 1053–64;. doi:10.1016/j.chembiol.2011.05.012. PMID 21867920.
- ↑ Lee, Y. J.; Choi, I. S.; Park, M. H.; Lee, Y. M.; Song, J. K.; Kim, Y. H.; Kim, K. H.; Hwang, D. Y.; Jeong, J. H.; Yun, Y. P.; Oh, K. W.; Jung, J. K.; Han, S. B.; Hong, J. T. (2011). "4-O-Methylhonokiol attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway". Free Radical Biology and Medicine. 50 (1): 66–77. doi:10.1016/j.freeradbiomed.2010.10.698. PMID 20974250.
- ↑ Gertsch, Anavi-Goffer (2012). "Methylhonokiol attenuates neuroinflammation: a role for cannabinoid receptors?". Journal of Neuroinflammation. 9 (135): 1053–64;. doi:10.1186/1742-2094-9-135.
- ↑ Chicca, A.; Gachet, M. S.; Petrucci, V.; Schuehly, W.; Charles, R. -P.; Gertsch, J. R. (2015). "4′-O-methylhonokiol increases levels of 2-arachidonoyl glycerol in mouse brain via selective inhibition of its COX-2-mediated oxygenation". Journal of Neuroinflammation. 12. doi:10.1186/s12974-015-0307-7.