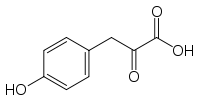
4-Hydroxyphenylpyruvic acid
 | |
| Names | |
|---|---|
| IUPAC name
3-(4-hydroxyphenyl)-2-oxo-propanoic acid | |
| Other names
4-Hydroxyphenylpyruvate p-Hydroxyphenylpyruvic acid p-Hydroxyphenylpyruvate | |
| Identifiers | |
| 156-39-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15999 |
| ChEMBL | ChEMBL607712 |
| ChemSpider | 954 |
| DrugBank | DB07718 |
| ECHA InfoCard | 100.005.322 |
| 6629 | |
| KEGG | C01179 |
| PubChem | 979 |
| |
| |
| Properties | |
| C9H8O4 | |
| Molar mass | 180.157 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
4-Hydroxyphenylpyruvic acid (4-HPPA) is an intermediate in the metabolism of the amino acid phenylalanine. The aromatic side chain of phenylalanine is hydroxylated by the enzyme phenylalanine hydroxylase to form tyrosine. The conversion from tyrosine to 4-HPPA is in turn catalyzed by tyrosine aminotransferase.[1] Additionally, 4-HPPA can be converted to homogentisic acid which is one of the precursors to ochronotic pigment.[2]
It is an intermediary compound in the biosynthesis of scytonemin.
See also
References
- ↑ Brand, Larry; Harper, Alfred (1974). "Effect of glucagon on phenylalanine metabolism and phenylalanine-degrading enzymes in the rat". Biochemical Journal. 142 (2): 231–45. PMC 1168273
 . PMID 4155291.
. PMID 4155291. - ↑ Denoya, Claudio; Skinner, Deborah; Morgenstern, Margaret (September 1994). "A Streptomyces avermitilis gene encoding a 4-hydroxyphenylpyruvic acid dioxygenase-like protein that directs the production of homogentisic acid and an ochronotic pigment in Escherichia coli". Journal of Bacteriology. 1 (17): 5312–5319. Retrieved 18 July 2011.
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.