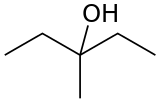
3-Methyl-3-pentanol
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylpentan-3-ol | |
| Other names
3-Methyl-3-pentanol Diethyl carbinol | |
| Identifiers | |
| 77-74-7 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL506184 |
| ChemSpider | 6248 |
| ECHA InfoCard | 100.000.959 |
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.174 g/mol |
| Appearance | colorless liquid |
| Odor | fruity |
| Density | 0.8286 g/cm3 at 20 °C |
| Melting point | −23.6 °C (−10.5 °F; 249.6 K) |
| Boiling point | 122.4 °C (252.3 °F; 395.5 K) |
| 45 g/L | |
| Solubility | miscible with ethanol, diethyl ether |
| Thermochemistry | |
| 293.4 J·mol−1·K−1 (liquid) | |
| Hazards | |
| Safety data sheet | http://www.sciencelab.com/msds.php?msdsId=9926087 |
| Related compounds | |
| Related compounds |
Hexanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
3-Methyl-3-pentanol (IUPAC name: 3-methylpentan-3-ol) is an organic chemical compound and a tertiary hexanol. It is used in the synthesis of the tranquilizer emylcamate.[2]
Synthesis
It can be prepared by reacting ethylmagnesium bromide with ethyl acetate in the so called Grignard reaction using dried diethyl ether or tetrahydrofuran as solvent.
It can be prepared also by reacting ethylmagnesium bromide with butanone in the same conditions already mentioned.
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–400, 5–47, 8–106, ISBN 0-8493-0594-2
- ↑ Sittig, Marshall (1988), Pharmaceutical manufacturing encyclopedia, 2 (2 ed.), William Andrew, pp. 555–556, ISBN 978-0-8155-1144-1, retrieved 2010-01-22
This article is issued from Wikipedia - version of the 10/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.