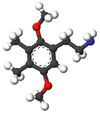
2C-G
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
3,4-Dimethyl-2,5-dimethoxyphenethylamine | |||
| Identifiers | |||
| 207740-18-9 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEMBL | ChEMBL127202 | ||
| ChemSpider | 21106224 | ||
| PubChem | 22238091 | ||
| |||
| |||
| Properties | |||
| C12H19NO2 | |||
| Molar mass | 209.28 g/mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
2C-G is a psychedelic phenethylamine of the 2C family. First synthesized by Alexander Shulgin, it is sometimes used as an entheogen. It has structural and pharmacodynamic properties similar to 2C-D and Ganesha. Like many of the phenethylamines in PiHKAL (Phenethylamines i Have Known And Loved), 2C-G and its homologues have only been taken by Shulgin and a small test group, making it difficult to ensure completeness when describing effects.
Chemistry
2C-G is 3,4-dimethyl-2,5-dimethoxyphenethylamine, with the formula C12H19NO2.
Dosage and effects
In Shulgin's book PiHKAL, the dosage range is listed as 20 to 35 mg. Effects are similar to the related Ganesha, and are extremely long lasting; the duration is 18–30 hours. Visual effects are muted or absent, and it is described in PiHKAL as an "insight-enhancer".[1] Unlike other members of the 2C family, 2C-G is nearly as potent as its amphetamine cousin.
Homologues
Several homologues of 2C-G were also synthesized by Shulgin. These include 2C-G-3, 2C-G-5, and 2C-G-N. Some, such as 2C-G-1, 2C-G-2, 2C-G-4, and 2C-G-6 are possible to synthesize in principle but impossible or extraordinarily difficult to do so in practice.
| 2C-G-1 | CAS: not available The synthesis of this compound has not been reported |
 2C-G-1 |
| 2C-G-2 | CAS: not available The synthesis of this compound has not been reported |
 2C-G-2 |
| 2C-G-3 | CAS: 207740-19-0 Dosage: 16–25 mg |
 2C-G-3 |
| 2C-G-4 | CAS: 952006-59-6 Synthesized but not tested. |
 2C-G-4 |
| 2C-G-5 | CAS: 207740-20-3 Dosage: 10–16 mg |
 2C-G-5 |
| 2C-G-6 | CAS: not available The synthesis of this compound has not been reported |
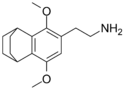 2C-G-6 |
| 2C-G-N | CAS: 207740-21-4 Dosage: 20–40 mg |
Law
2C-G and all of its homologues are unscheduled and uncontrolled in the United States, but possession and sales of 2C-G (and homologues) will probably be prosecuted under the Federal Analog Act because of their structural similarities to 2C-B.
Canada
As of October 31st, 2016; 2C-G is a controlled substance (Schedule III) in Canada. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php
United Kingdom
2C-G and all other compounds featuring in PiHKAL are Class A drugs in the United Kingdom.
See also
References
- ↑ Shulgin, Alexander; Ann Shulgin (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
External links
- PiHKAL entry on 2C-G
- ,,, PiHKAL entries on the homologues
- 2C-G Entry in PiHKAL • info
- 2C-G-3 Entry in PiHKAL • info
- 2C-G-4 Entry in PiHKAL • info
- 2C-G-5 Entry in PiHKAL • info
- 2C-G-N Entry in PiHKAL • info