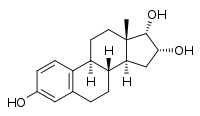
17α-Epiestriol
 | |
| Names | |
|---|---|
| IUPAC name
(1S,10R,11S,13R,14S,15S)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-triene-5,13,14-triol | |
| Other names
17-Epiestriol; 16α-Hydroxy-17α-estradiol; Estra-1,3,5(10)-triene-3,16α,17α-triol; 3,16α,17α-Trihydroxy-1,3,5(10)-estratriene | |
| Identifiers | |
| 1228-72-4 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1232445 |
| ChemSpider | 225189 |
| DrugBank | DB07702 |
| PubChem | 256737 |
| UNII | 4G7IHY560Z |
| |
| |
| Properties | |
| C18H24O3 | |
| Molar mass | 288.38136 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiol or estra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimer of estriol (which is 16α-hydroxy-17β-estradiol).[1][2][3] It is formed from 16α-hydroxyestrone.[4][5] In contrast to other endogenous estrogens like estradiol, 17α-epiestriol is a selective agonist of the ERβ.[6] It is described as a relatively weak estrogen, which is in accordance with its relatively low affinity for the ERα.[7] 17α-Epiestriol has been found to be approximately 400-fold more potent than estradiol in inhibiting tumor necrosis factor α (TNFα)-induced vascular cell adhesion molecule 1 (VCAM-1) expression in vitro.[8]
See also
References
- ↑ Ashutosh K Tewari (5 April 2013). Prostate Cancer: A Comprehensive Perspective. Springer Science & Business Media. pp. 373–. ISBN 978-1-4471-2864-9.
- ↑ A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 522–. ISBN 978-3-642-96158-8.
- ↑ N. S. Assali (3 September 2013). The Maternal Organism. Elsevier. pp. 341–. ISBN 978-1-4832-6380-9.
- ↑ U.S. Von Euler (2 December 2012). Comparative Endocrinology. Elsevier Science. pp. 135–. ISBN 978-0-323-14609-8.
- ↑ Norbert W. Tietz (1 August 1976). Fundamentals of clinical chemistry. Saunders. p. 773. ISBN 978-0-7216-8866-4.
- ↑ Gajanan V. Sherbet (26 July 2013). Therapeutic Strategies in Cancer Biology and Pathology. Elsevier. pp. 83–. ISBN 978-0-12-416590-8.
- ↑ Ralph I. Dorfman (22 October 2013). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 13–. ISBN 978-1-4832-7299-3.
- ↑ Mukherjee, T. K.; Nathan, L.; Dinh, H.; Reddy, S. T.; Chaudhuri, G. (2003). "17-Epiestriol, an Estrogen Metabolite, Is More Potent Than Estradiol in Inhibiting Vascular Cell Adhesion Molecule 1 (VCAM-1) mRNA Expression". Journal of Biological Chemistry. 278 (14): 11746–11752. doi:10.1074/jbc.M207800200. ISSN 0021-9258.
This article is issued from Wikipedia - version of the 12/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.