1,2-Diaminopropane
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Propanediamine | |
| Systematic IUPAC name
Propane-1,2-diamine | |
| Identifiers | |
| 78-90-0 | |
| 3D model (Jmol) | Interactive image |
| 605274 | |
| ChEBI | CHEBI:30630 |
| ChemSpider | 13849260 394801 R 557530 S |
| ECHA InfoCard | 100.001.051 |
| EC Number | 201-155-9 |
| 25709 | |
| MeSH | 1,2-diaminopropane |
| PubChem | 6567 447820 R 642322 S |
| RTECS number | TX6650000 |
| UN number | 2258 |
| |
| |
| Properties | |
| C3H10N2 | |
| Molar mass | 74.13 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Fishy, ammoniacal |
| Density | 870 mg mL−1 |
| Melting point | −37.1 °C; −34.9 °F; 236.0 K |
| Boiling point | 119.6 °C; 247.2 °F; 392.7 K |
| Vapor pressure | 1.9 Pa (at 20 °C) |
| Refractive index (nD) |
1.446 |
| Thermochemistry | |
| 205.64 J K−1 mol−1 | |
| Std molar entropy (S |
247.27 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
−98.2–−97.4 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−2.5122–−2.5116 MJ mol−1 |
| Hazards | |
| GHS pictograms |    |
| GHS signal word | DANGER |
| H226, H302, H312, H314 | |
| P280, P305+351+338, P310 | |
| EU classification (DSD) |
|
| R-phrases | R10, R21/22, R35 |
| S-phrases | S26, S37/39, S45 |
| Flash point | 34 °C (93 °F; 307 K) |
| 360 °C (680 °F; 633 K) | |
| Explosive limits | 1.9–11.1% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
|
| Related compounds | |
| Related alkanamines |
|
| Related compounds |
2-Methyl-2-nitrosopropane |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
1,2-Diaminopropane (1,2-propanediamine) is a diamine commonly used as a bidentate ligand in coordination chemistry. Pn is the simplest chiral diamine. The molecules exists as a colorless liquid at room temperature.
Preparation
Industrially, this compound is synthesized by the ammonolysis of 1,2-dichloropropane:[1] This preparation allows for the use of waste chloro-organic compounds to form useful amines using inexpensive and readily available ammonia:[1]
- CH3CHClCH2Cl + 4 NH3 → CH3CH(NH2)CH2NH2 + 2 NH4Cl
The racemic mixture of this chiral compound may be separated into enantiomers by conversion into the diastereomeric tartaric acid ammonium salt. After purification of the diastereomer, the diamine can be regenerated by treatment of the ammonium salt with sodium hydroxide.[2] Alternate reagents for chiral resolution include N-p-toluenesulfonylaspartic acid, N-benzenesulfonylaspartic acid, or N-benzoylglutamic acid.[3]
N,N-Disalicylidene-1,2-propanediamine
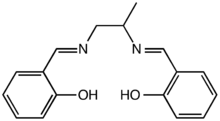
1,2-Diaminopropane can be converted to N,N′-disalicylidene-1,2-propanediamine, a useful salen-type ligand that is abbreviated salpn. The synthesis is achieved by a condensation reaction of the diamine with salicylaldehyde:
- 2C6H4(OH)CHO + CH3CH(NH2)CH2NH2 → [C6H4(OH)CH]2CH3CHNCH2N + 2H2O
Salpn is used as a fuel additive as a metal deactivator in motor oils. Trace metals degrade the fuels by catalyzing oxidation processes that lead to gums and solids. Metal deactivators like salpn form stable complexes with the metals, suppressing their catalytic activity.[4] While salpn forms stable chelate complexes with many metals including copper, iron, chromium, and nickel, it is the coordination with copper that makes it a popular choice as a fuel additive. Copper has the highest catalytic activity in fuel, and salpn forms a highly stable square-planar complex with the metal. This complex is especially stable because salpn is a tetra-dentate ligand with a -2 charge (after deprotonation of the two phenolic groups).[5]
The use of salpn is preferred over the use of ethylenediamine (producing salen), possibly because the propane derivatives has higher solubility.
References
- 1 2 Bartkowiak, M.; Lewandowski, G.; Milchert, E.; Pelech, R. (2006). "Optimization of 1,2-Diaminopropane Preparation by the Ammonolysis of Waste 1,2-Dichloropropane". Ind. Eng. Chem. Res. 45: 5681–5687. doi:10.1021/ie051134u.
- ↑ Romanowski, G.; Wera, M. (2010). "Mononuclear and dinuclear chiral vanadium(V) complexes with tridentate Schiff bases derived from R(−)-1,2-diaminopropane: Synthesis, structure, characterization and catalytic properties". Polyhedron. 29: 2747–2754. doi:10.1016/j.poly.2010.06.030.
- ↑ JP application 04-018057, Sakie, N. & Haruyo, S., "Production of Optically Active 1,2-propanediamine"
- ↑ Dabelstein, W.; Reglitzky A.; Schutze A.; Reders, K. (2005), "Automotive Fuels", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH
- ↑ Evans, D. A.; Miller, S. J.; Lectka, T.; von Matt. P. (1999). "Chiral Bis(oxazoline)copper(II) Complexes as Lewis Acid Catalysts for Enantioselective Diels-Alder Reaction". J. Am. Chem. Soc. 121: 7559–7573. doi:10.1021/ja991190k.