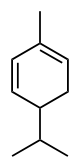
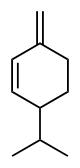
Phellandrene
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
α: 2-Methyl-5-(1-methylethyl)-1,3-cyclohexadiene β: 3-Methylene-6-(1-methylethyl)cyclohexene | |||
| Identifiers | |||
| 99-83-2 (α) 555-10-2 (β) | |||
| 3D model (Jmol) | (α): Interactive image (β): Interactive image | ||
| ChemSpider | 7180 (α) 10669 (β) | ||
| |||
| |||
| Properties[1] | |||
| C10H16 | |||
| Molar mass | 136.24 g/mol | ||
| Appearance | Colorless oil (α and β) | ||
| Density | α: 0.846 g/cm3 β: 0.85 g/cm3 | ||
| Boiling point | α: 171-172 °C β: 171-172 °C | ||
| Insoluble (α and β) | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Phellandrene is the name for a pair of organic compounds that have a similar molecular structure and similar chemical properties. α-Phellandrene and β-phellandrene are cyclic monoterpenes and are double-bond isomers. In α-phellandrene, both double bonds are endocyclic and in β-phellandrene, one of them is exocyclic. Both are insoluble in water, but miscible with ether.
α-Phellandrene was named after Eucalyptus phellandra, now called Eucalyptus radiata, from which it can be isolated.[2] It is also a constituent of the essential oil of Eucalyptus dives.[3] β-Phellandrene has been isolated from the oil of water fennel and Canada balsam oil.
The phellandrenes are used in fragrances because of their pleasing aromas. The odor of β-phellandrene has been described as peppery-minty and slightly citrusy.
The α-phellandrene isomer can form hazardous and explosive peroxides on contact with air at elevated temperatures.[4]
References
- ↑ The Merck Index, 12th Edition, 7340, 7341
- ↑ Jacobs, S.W.L., Pickard, J., Plants of New South Wales, 1981, ISBN 0-7240-1978-2.
- ↑ Boland, D.J., Brophy, J.J., and A.P.N. House, Eucalyptus Leaf Oils, 1991, ISBN 0-909605-69-6.
- ↑ Urben, Peter (2007). Bretherick's Handobook of Reactive Chemical Hazards. 1 (7 ed.). Butterworth-Heinemann. p. 1154.